Lymph Node Transfer Reduces Lymphoedema After Breast Cancer Surgery

A multicentre study led from Finland has shown that lymph node transfer is a viable treatment for the swelling in the affected limb, a condition known as lymphoedema, after breast cancer surgery. Unfortunately, a drug to improve the outcomes of the transfer treatment was not shown to be effective.
“I am becoming increasingly convinced that lymphoedema is not just a lymphatic problem, but is connected to an immunological factor,” says Plastic Surgeon and InFLAMES Flagship Researcher Pauliina Hartiala from the University of Turku in Finland.
The study by Hartiala and collaborators was published in the journal Plastic and Reconstructive Surgery.
Around one in four women with breast cancer undergo an axillary lymph node removal surgery. The surgery is performed if tests show that the cancer has spread from the breast tissue to the lymph nodes and is often followed by radiotherapy.
After the treatment, around 20–40% of women develop lymphoedema, a lymphatic drainage disorder in the affected arm. In 2022, about 2.3 million women were diagnosed with breast cancer worldwide. In men, the disease is rare.
Swelling can start years after treatment
“Lymphedema usually starts about six months after cancer surgery, but can also occur with a delay of several years after the cancer has been treated,” says Pauliina Hartiala.
In lymphedema, fluid accumulates in the tissue at first, but over time fat and firm connective tissue also begin to accumulate in the arm. Eventually, the upper limb becomes thick and clumsy. An elastic compression sleeve is used to try to control the problem by applying pressure to prevent the limb from swelling. However, the swelling can become so severe that the arm clearly interferes with everyday life, both at work and at leisure.
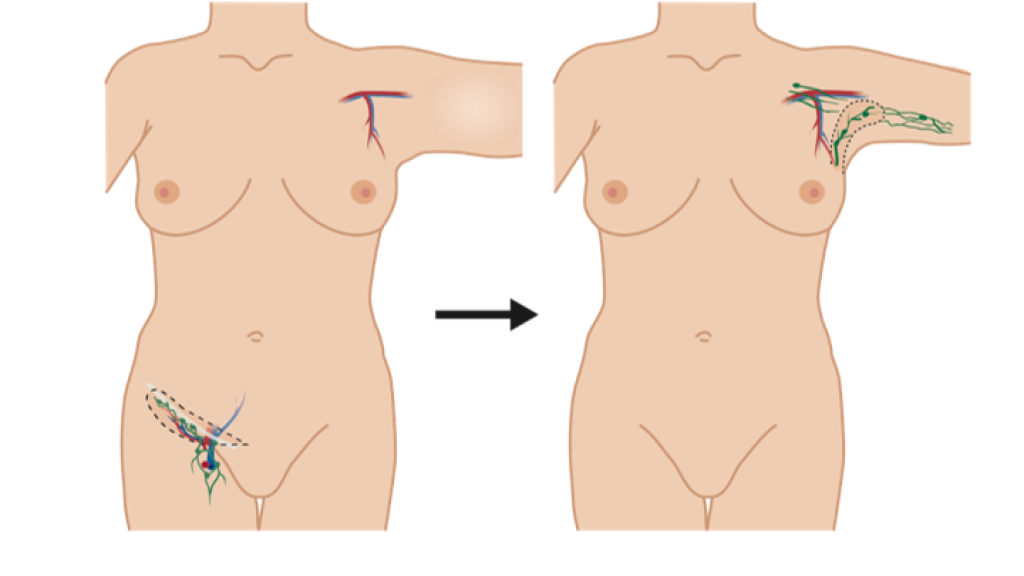
Lymphedema can be treated with surgical options including liposuction, lymphatic bypass procedure, or lymph node transfer. In the transfer surgery, the patient’s lymph nodes are transferred from the groin area to the armpit, or axilla, to replace the removed lymph nodes. The procedure involves extensive scar removal from the armpit.
Lymph node transfer is often performed at the same time as the breast operated on for cancer is reconstructed with a tissue flap taken from the patient’s abdomen.
Additional benefits were expected from a growth factor
Pauliina Hartiala was one of the leaders of a multicentre study in Turku, Finland, focused on investigating whether the outcome of lymph node transfer could be improved by a growth factor, a drug called Lymfactin, that promotes growth and repair of lymphatic vessels. The study was carried out in five research centres in Finland and Sweden and builds on extensive basic research led by Finnish Professors Kari Alitalo, Seppo Ylä-Herttuala and Anne Saarikko. Lymfactin is a research product from the Finnish pharmaceutical company Herantis Pharma.
The study tested whether the growth factor could improve lymph node flap function compared to a lymph node transfer conducted without the drug. The study involved 39 women. Of these, 20 underwent a transfer procedure where the tissue flap was injected with the lymphatic growth factor before it was transferred. For the second group, the transfer was carried out by adding only saline (placebo) to the tissue flap.
“Even though the drug therapy had worked well in combination with lymph node transfer in the animal model, it did not provide sufficient additional benefit to surgery in humans,” says Pauliina Hartiala.
Although Lymfactin did not work as expected in humans, Pauliina Hartiala is pleased with the other results of the study. In both study groups, the excess arm volume reduced during follow-up. In addition, the patients treated with Lymfactin had a significantly greater reduction in skin interstitial fluid than the placebo group.
“We are the first to show, with a double-blind study, that lymph node transfer is a viable treatment for some patients with lymphoedema after breast cancer surgery. One of the results of our study was the fact that the operation significantly improved women’s quality of life, which is an important finding.”
Pauliina Hartiala works as a Plastic Surgeon at Turku University Hospital, alongside her research work. She now believes that besides a lymphatic problem, lymphedema is linked to an immunological factor. It may be one or more of the immune cells that are involved in the accumulation of connective tissue and fat in the lymphoedema.
“If this is the case, further research will allow us to investigate whether regulating the functions of this cell population could reduce fat accumulation in the limb,” concludes Hartiala.
Source: University of Turku