When Should Preventive Mastectomy be Offered in High Breast Cancer Risk?
More women at higher risk of breast cancer should be offered a mastectomy, according to researchers at Queen Mary and London School of Hygiene and Tropical Medicine.
A new analysis, published in JAMA Oncology, has found that the surgical technique was a cost-effective way of reducing the likelihood of developing breast cancer compared to breast screening and medication. Current guidelines on who is offered mastectomy may need to be revised to reflect these new findings.
Clinicians currently use personalised risk prediction models which combine genetic and other data to identify those women who are at a higher risk of developing breast cancer (BC). Subsequent treatment options – including mammograms, MRI screening, surgery, and medication – are then offered dependent on each woman’s level of risk.
Risk reducing mastectomy (RRM) is recommended for women at high risk, but in practice this surgery is only clinically offered to those carrying faults (called pathogenic variants) in genes that are known to increase the likelihood they will develop the disease (BRCA1/ BRCA2/ PALB2 PV).
Professor Ranjit Manchanda from Queen Mary University of London, Dr Rosa Legood from London School of Hygiene and Tropical Medicine, along with colleagues from Manchester University and Peking University created a new economic evaluation model to accurately predict the level of risk that would make RRM a more cost-effective treatment.
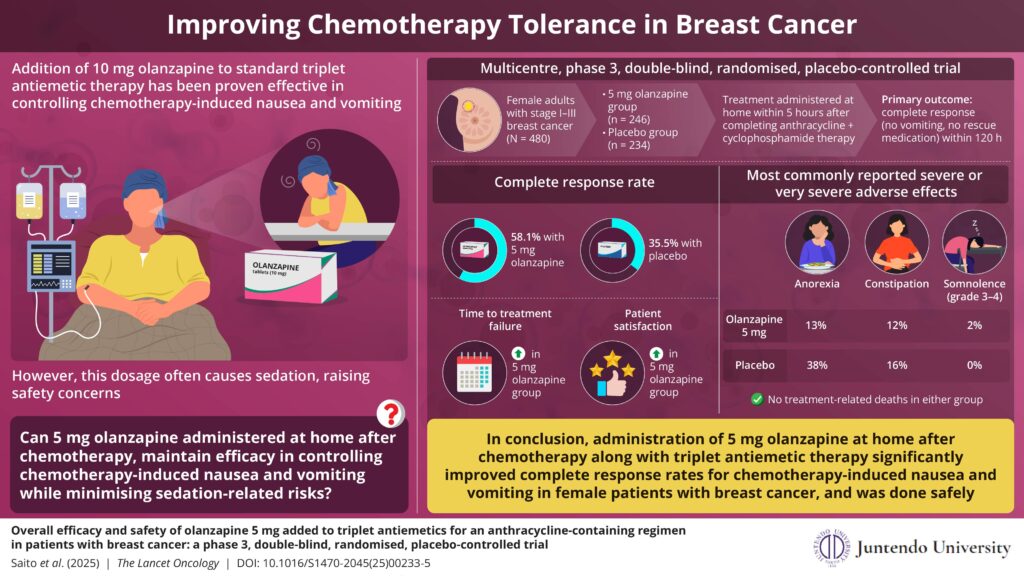
For their model, researchers used guidelines from the National Institute for Health and Care Excellence (NICE) to determine whether a treatment is considered cost-effective. Their model showed that mastectomy was a cost-effective treatment for women aged 30 or above who have a lifetime breast cancer risk greater than or equal to 35%. Offering RRM to women in this cohort could potentially prevent 6,500 of the 58,500 cases of breast cancer that are diagnosed every year in the UK.
Professor Manchanda, Professor of Gynaecological Oncology at Queen Mary and Consultant Gynaecological Oncologist, said: “We for the first time define the risk at which we should offer RRM. Our results could have significant clinical implications to expand access to mastectomy beyond those patients with known genetic susceptibility in high penetrance genes- BRCA1/ BRCA2/ PALB2 – who are traditionally offered this. This could potentially prevent can potentially prevent ~6500 breast cancer cases annually in UK women. We recommend that more research is carried out to evaluate the acceptability, uptake, and long-term outcomes of RRM among this group”..
Dr Legood, Associate Professor in health economics at the London School of Hygiene & Tropical Medicine, said: “Undergoing RRM is cost-effective for women 30-55 years with a lifetime breast cancer risk of 35% or more. These results can support additional management options for personalized breast cancer risk prediction enabling more women at increased risk to access prevention.”
Dr Vineeth Rajkumar, Head of Research at Rosetrees, said: “Rosetrees is delighted to fund this truly groundbreaking research that could have a positive impact on women worldwide.”
The researchers used data from women aged between 30 and 60 with varying lifetime breast cancer risks between 17% and 50%, and who were either undergoing RRM or receiving screening with medical prevention according to currently used predictive models.
NICE deems a treatment cost-effective if it typically brings one additional year of health for no more than £20 000–£30 000 per patient (known as the ‘willingness to pay’ threshold, or WTP). The researchers’ model used a threshold of £30 000/Quality Adjusted Life Year.
Source: Queen Mary University London