Chemotherapy’s Gut Side Effects Unexpectedly Block Metastasis
Ludivine Bersier 2025
In Nature Communications, researchers from Lusanne University reveal that chemotherapy alters gut microbes and bone marrow immune cell development, unexpectedly reprogramming systemic immunity in ways that help restrict metastatic progression.
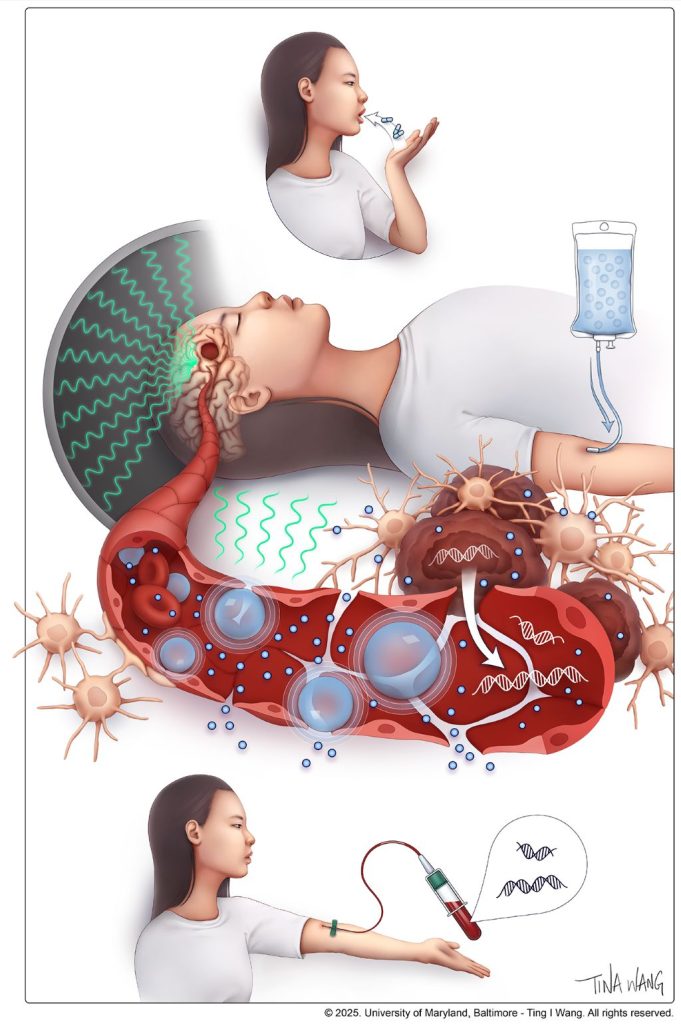
Chemotherapy commonly damages the intestinal lining, a well-known side effect. But this injury does not remain confined to the gut. It reshapes nutrient availability for intestinal bacteria, forcing the microbiota to adapt.
The researchers report that chemotherapy-induced damage to the intestinal lining alters nutrient availability for gut bacteria, reshaping the microbiota and increasing the production of indole-3-propionic acid (IPA), a tryptophan-derived microbial metabolite.
Rather than acting locally, IPA functions as a systemic messenger. It travels from the gut to the bone marrow, where it rewires immune cell production. Elevated IPA levels reprogram myelopoiesis, reducing the generation of immunosuppressive monocytes that facilitate immune evasion and metastatic growth.
“We were surprised by how a side effect often seen as collateral damage of chemotherapy can trigger such a structured systemic response. By reshaping the gut microbiota, chemotherapy sets off a cascade of events that rewires immunity and makes the body less permissive to metastasis.” says Ludivine Bersier, first author of the study.
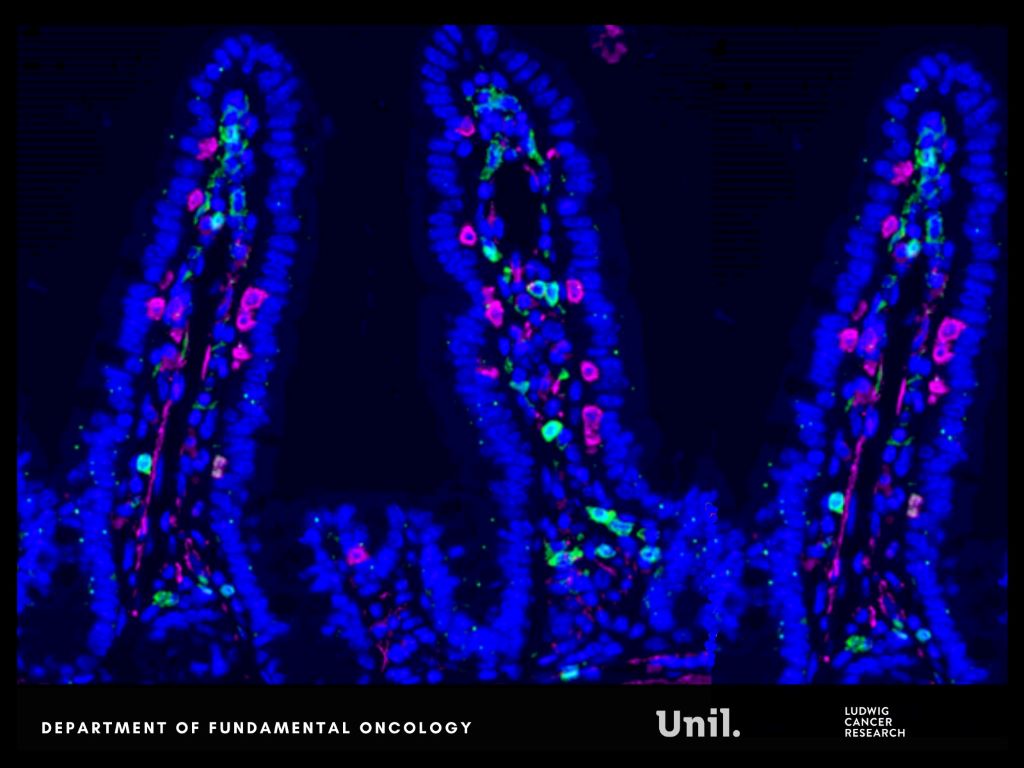
This immune reconfiguration enhances T-cell activity and remodels immune interactions within metastatic niches, particularly in the liver, resulting in a metastasis-refractory state in preclinical models.
Experimental findings are mirrored in patients. Clinical relevance is supported by patient data obtained in collaboration with Dr Thibaud Koessler (Geneva University Hospitals, HUG). In patients with colorectal cancer, higher circulating IPA levels following chemotherapy are associated with reduced monocyte levels, a feature of improved survival outcomes.
“This work shows that the effects of chemotherapy extend far beyond the tumor itself. By uncovering a functional axis linking the gut, the bone marrow and metastatic sites, we highlight systemic mechanisms that could be harnessed to durably limit metastatic progression.” says Tatiana Petrova, corresponding author of the study.
This research was supported by multiple funders, including the Swiss National Science Foundation and Swiss Cancer League. An ISREC Foundation Tandem Grant supported close collaboration between clinical and fundamental research, led at Unil by Professor Tatiana Petrova and Dr Thibaud Koessler at HUG. The project posits that chemotherapy can induce a form of biological “memory”, mediated by gut microbiome–derived metabolites that durably inhibit metastatic growth.
Together, these findings reveal a previously underappreciated gut–bone marrow–liver metastasis axis through which chemotherapy can exert durable systemic effects, opening new avenues to harness microbiota-derived metabolites as adjuvant strategies to limit metastasis.
Source: EurekAlert!, University of Lusanne