The Power of Touch: Skin-to-skin Contact Linked to Preemie Brain Growth
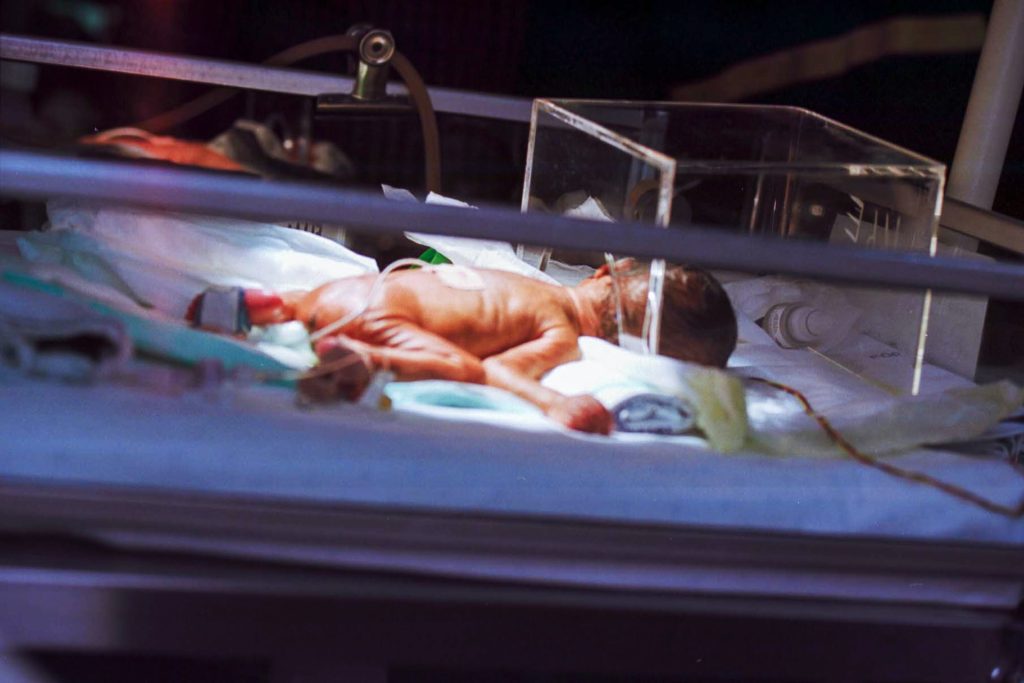
Preterm infants born before 32 weeks who received more skin-to-skin contact while in the hospital showed stronger brain development in areas tied to emotion and stress regulation than babies who received less skin-to-skin care, according to a study published in Neurology®, the medical journal of the American Academy of Neurology. The study can only show an association and cannot establish causation.
“Skin-to-skin contact in preterm infants has been shown to have many benefits, with previous studies linking it to improved bonding, sleep, heart and lung function and growth, as well as reduced pain and stress,” said study author Katherine E. Travis, PhD, of Burke Neurological Institute in White Plains, New York. “Our findings in infants born very preterm suggest skin-to-skin care may also play a role in shaping early brain development, highlighting the potential importance of caregiving experiences during the earliest weeks of a preemie’s life.”
he study included 88 preterm infants with an average gestational age of 29 weeks who weighed an average of 2.65 pounds. The average stay in the hospital was two months. The goal was to find out whether skin-to-skin holding, also called kangaroo care, was linked to brain development in areas that help regulate emotions and stress. Researchers tracked skin-to-skin care with family members throughout each infant’s hospitalisation, including how long each session lasted and the total minutes per day. Families visited an average of once per day. When they provided skin-to-skin care, the average session was around 70 minutes with 73% of sessions provided by mothers. For the entire hospital stay, the average amount of skin-to-skin care per day was 24 minutes.
Each infant received a brain scan before going home from the hospital – around the time they would have reached full-term age of around 40 weeks. The brain scans measured how water moves through brain tissue. This movement helps reveal how white matter – the brain’s communication network – is developing. Researchers then compared the markers of white matter with the amount of time the preemies received skin-to-skin care per session and per day.
For skin-to-skin duration per session, researchers found longer sessions were linked to higher mean diffusivity – how freely water moves through the brain – in two key brain regions: the cingulum, which supports attention and emotion regulation; and the anterior thalamic radiations, which connects areas involved in emotional processing and memory.
Longer sessions were also linked to lower fractional anisotropy – how water movement is influenced by developing cellular tissues – in the anterior thalamic radiations. For daily total minutes of skin-to-skin care, researchers found higher amounts were linked to higher mean diffusivity in the anterior thalamic radiations. They were also linked to lower fractional anisotropy in the anterior thalamic radiations. These associations remained significant even after researchers accounted for factors that could influence brain development, including gestational age at birth, age at time of scan, socioeconomic status and how often family visited.
“Our findings add to growing evidence that white matter development is sensitive to a preterm infant’s experience while in the hospital,” said Travis. “Skin-to-skin care not only provides preterm infants with family connections through bonding, it may also be encouraging new connections within the brain itself, improving a baby’s brain health overall.”
A limitation of the study is that it was conducted at a single hospital and researchers reviewed existing medical records. The authors note that future research should explore how early caregiving experiences – like skin-to-skin care – might shape brain development and support later behavioural outcomes as preterm infants grow.
Source: American Academy of Neurology