New Ultrasound Helmet Enables Deep Brain Stimulation in People Without Surgery
Scientists have long been looking for a way to modulate brain function, which could improve our understanding of how the brain works and help to treat neurological diseases, using non-invasive methods that don’t involve surgery.
One technology that could help is transcranial ultrasound stimulation (TUS), which was recently discovered to be able to modulate the activity of neurons (the brain’s key communication cells) by delivering gentle mechanical pulses that influence how these cells send signals.
But to date current systems have struggled to reach deeper areas of the brain with sufficient precision to target specific brain structures. Conventional TUS systems often affect broader regions than intended, limiting their utility for targeted neuromodulation.
The study, published in Nature Communications, introduces a new ultrasound device capable of influencing deep brain regions without surgery for the first time, targeting areas around 1000 times smaller than conventional ultrasound devices can pinpoint and 30 times smaller than previous deep brain ultrasound devices.
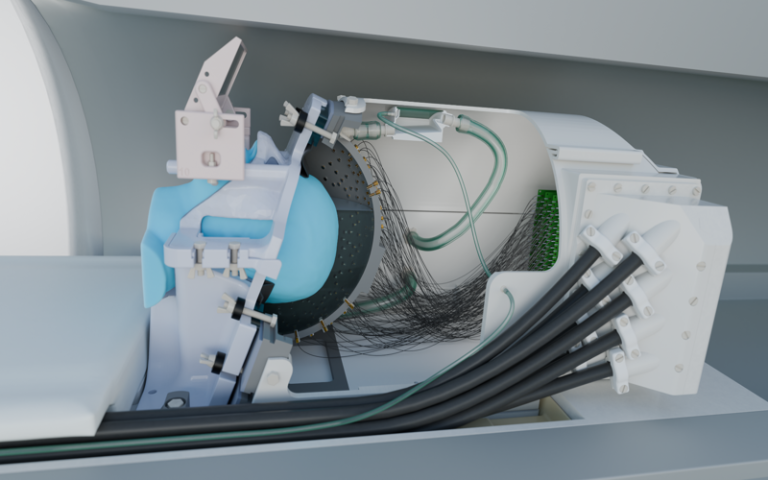
The new technology features 256 elements configured within a special helmet to send focused beams of ultrasound to specific parts of the brain in order to turn neuronal activity up or down. It also includes a soft plastic face mask which helps to target the ultrasound waves more precisely by keeping the head still.
The research team demonstrated the system’s capabilities on seven human volunteers by targeting a part of the thalamus, a small structure in the centre of the brain that helps to relay sensory and motor information, called the lateral geniculate nucleus (LGN). The LGN is involved in processing visual information.
In the first experiment, participants looked at a flashing checkerboard, which sent signals to the brain through the eyes. During stimulation with the ultrasound device, a functional magnetic resonance imaging (fMRI) scan showed significantly increased activity in the participants’ visual cortex, confirming precise targeting of the LGN.
A second experiment revealed sustained decreases in visual cortex activity for at least 40 minutes after ultrasound stimulation, highlighting the system’s potential for inducing lasting changes in brain function.
Though participants did not consciously perceive any changes in what they were seeing during the experiments, the brain scans revealed significant changes in neural activity. The ultimate goal is to harness these effects to produce clinically beneficial outcomes, such as stopping hand tremors.
Professor Bradley Treeby, senior author of the study from UCL Medical Physics and Biomedical Engineering, said: “This advance opens up opportunities for both neuroscience research and clinical treatment. For the first time, scientists can non-invasively study causal relationships in deep brain circuits that were previously only accessible through surgery.
“Clinically, this new technology could transform treatment of neurological and psychiatric disorders like Parkinson’s disease, depression, and essential tremor, offering unprecedented precision in targeting specific brain circuits that play key roles in these conditions.
“The ability to precisely modulate deep brain structures without surgery represents a paradigm shift in neuroscience, offering a safe, reversible, and repeatable method for both understanding brain function and developing targeted therapies.”
In addition to its research applications, the system could pave the way for new clinical interventions. Deep brain stimulation (DBS), currently used to treat conditions like Parkinson’s disease, requires invasive surgery and carries associated risks. The new ultrasound system offers a non-invasive alternative with comparable precision, potentially allowing clinicians to test areas of the brain that could be used to treat disease before surgery or even replace surgical approaches altogether.
Recognising this clinical potential, several members of the research team have recently founded NeuroHarmonics, a UCL spinout company developing a portable, wearable version of the system. The company aims to make precise, non-invasive deep brain therapy accessible for both clinical treatment and broader therapeutic applications.
Dr Eleanor Martin, first author of the study from UCL Medical Physics and Biomedical Engineering, said: “We designed the system to be compatible with simultaneous fMRI, enabling us to monitor the effects of stimulation in real time. This opens up exciting possibilities for closed-loop neuromodulation and personalised therapies.”
The researchers emphasise that further studies are needed to fully understand the mechanisms underlying TUS-induced neuromodulation. However, the results mark a significant milestone in the development of safe, effective, and targeted brain stimulation technologies.
Source: University College London