Antibiotic Slashes Risk of Drug-resistant TB in Kids, Finds Major SA Study

By Elri Voigt for Spotlight
For decades, the standard way to prevent people who were exposed to tuberculosis (TB) from falling ill with the disease was to offer them a medicine called isoniazid, taken daily for six or more months. That changed in the last decade with the development of new preventive therapy regimens that are taken for four, three, or even just one month.
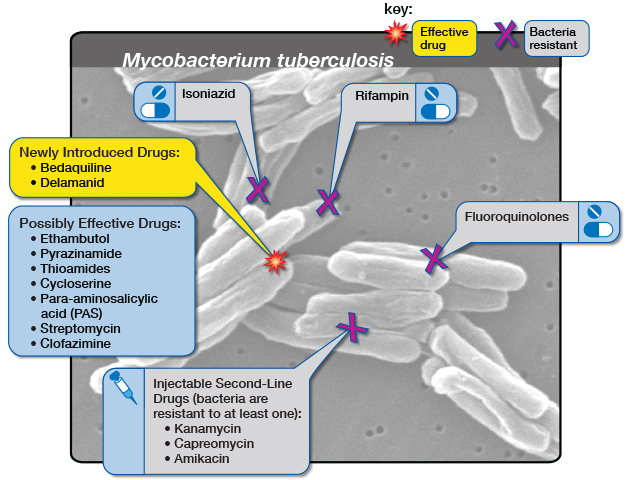
One complexity, however, is that both isoniazid and the new regimens are much better at preventing normal drug-sensitive TB than they are at preventing drug-resistant forms of TB. This is not surprising. As explained by Paediatric Infectious Disease doctor and Professor of Global Child Health at Imperial College London, Dr James Seddon, the two drugs that have mainly been used to prevent drug-susceptible TB are isoniazid and rifampicin (rifampicin’s sister drug rifapentine is also used). Now, by definition, he explains multidrug-resistant (MDR) TB is resistant to both these drugs so it’s unlikely to have any impact.
The situation is particularly tricky when it comes to children. In a 2020 statement the World Health Organization (WHO) says that it estimated that worldwide between 25 000 and 32 000 children develop MDR-TB each year, and mainly acquire it through transmission from close contact with an adult or adolescent who has MDR-TB. According to Seddon, while there is some emerging observational evidence on the use of drugs other than isoniazid and rifampicin to prevent MDR-TB, there has been no clinically tested regimen to give to children following MDR-TB exposure.
Now, much anticipated results from a phase three trial has shown that a single antibiotic pill, given daily for six months, is safe and effective to use in children who have been exposed to MDR-TB.
Results from TB CHAMP
The trial, called TB-CHAMP, looked at the efficacy and safety of using the antibiotic levofloxacin to prevent TB in children exposed to MDR-TB. Top-line findings from the study was presented last week at the Union World Lung Conference held in Paris, France.
“The paediatric population is probably the most neglected of all the populations affected by MDR-TB,” Dr Anneke Hesseling, Director of the Paediatric TB Research Programme at Stellenbosch University, told the conference. “Fewer than 20% who develop MDR-TB disease are actually diagnosed and treated, and so to find more cases and prevent more cases is really, really critical…So prevention is really key, and the TB-CHAMP trial is really a phase three efficacy trial looking at levofloxacin to prevent new cases of TB in children and also looking at the safety of levofloxacin.”
Hesseling, who is the Principal Investigator of the study, says that TB-CHAMP is the first trial to provide clinical data on what drug might be used to prevent TB in children who have been exposed to MDR-TB. It was conducted at five sites across South Africa, all with high MDR-TB burdens. The study was led by Stellenbosch University and the Desmund Tutu TB Centre. The findings have not yet been published in a peer-reviewed journal.
922 children were randomised to receive either levofloxacin or a placebo for six months. 453 children got levofloxacin and 469 got the placebo. The primary efficacy data featured data from 916 of those children, with 451 in the levofloxacin arm and 465 in the placebo arm.
Hesseling says that only children who were exposed to an adult in their household with confirmed MDR-TB were included in the study. At first children below the age of five were recruited, regardless of their TB infection status. Later children between the ages of five and 17 were included, but they had to either have a TB infection or be living with HIV. The majority of the children, 90%, were younger than five years. TB infection was confirmed with a blood test.
By 48 weeks, Hesseling says five children in the levofloxacin arm versus 12 in the placebo arm developed TB, which amounts to an incidence rate of 1.1% in the levofloxacin arm, and 2.6% in the placebo arm.
Implication of results
“While TB preventive therapy (TPT) has long been recommended and available for young child contacts of people with drug-susceptible TB, there has not been sufficient evidence to make strong recommendations for treatment that could prevent DR-TB. Therefore, the TB-CHAMP findings are critically important for a number of reasons,” says Professor Guy Marks, President and Interim Executive Director, International Union Against Tuberculosis and Lung Disease (The Union).
“The study provides the first high-quality evidence that DR-TB can be prevented in children by using six months of daily levofloxacin, and that this is a safe medication. Furthermore, this will encourage more community-based contact screening, which will also lead to early detection of children and contacts of all ages who already have disease, and initiate treatment,” he adds.
“The impact [of the TB-CHAMP results] is potentially tremendous as it would prevent DR TB among child contacts. DR TB is more complex to treat and cure and often children are marginalised, so this study puts the spotlight on an effective way to protect children. This is not just about the life and health of the child but the social, economic and mental health implications for the caregiver and the entire family,” says Dr Priashni Subrayen, Technical Director for TB at The Aurum Institute.
Seddon, who is also one of the Co-PIs for the study, tells Spotlight that it was important to establish the safety of levofloxacin since it belongs to a class of drugs called the fluoroquinolones, which were thought to have terrible side effects when used in children.
Results from TB-CHAMP show that this is not the case.
The side effects were mild, and the regimen was well tolerated, according to Hesseling, with only eight children having a grade one or higher adverse event in the levofloxacin arm compared to four in the placebo arm. Two deaths were reported, one in each study arm, but were unrelated to the study. Overall, six children in the levofloxacin arm discontinued treatment or left the study early.
Researchers from TB-CHAMP collaborated with researchers from the V-QUIN trial – a phase three study that looked at levofloxacin as TB prevention in adults in Vietnam – in order to combine their data which allowed them to show data for levofloxacin across different age groups. Seddon explains: “They’ve applied a novel analytic approach, which uses a Bayesian, or probabilistic, framework, where we take the results of TB-CHAMP and we say well, if we actually use some of the information from V-QUIN to inform the TB-CHAMP results, we can make that a slightly more confident estimate,” he says.
The combined results, according to Hesseling were able to also show that levofloxacin reduced the risk of TB by about 60% across the age spectrum but with a tighter confidence interval, indicating a more precise estimate of the effect.
Seddon tells Spotlight that the combined data showed that there were no serious adverse events, but the adult population experienced more grade one and grade two side effects than the children, but these went away either over time or when the drug was stopped. The side effects included inflammation in the joints and tendons, which is a known side effect of this class of drug.
Not a silver bullet
While the findings could be a game-changer and potentially inform MDR-TB prevention guidelines, particularly in children, the regimen is by no means a silver bullet. Seddon says that while the regimen was safe, when participants were asked whether they liked the medicine, more people said they didn’t like it in the levofloxacin group versus the placebo. Another downside is that the pill was an adult formulation and thus needed to be cut and/or crushed for the kids to swallow.
Seddon explains that the WHO, who have been provided with the data from both studies and expected to meet in early December, would need to consider a variety of factors before deciding what to recommend about the use of levofloxacin for prevention. That includes the fact that you need to treat a lot of children for six months who might not have TB despite being exposed in order to prevent a few cases.
“You have to weigh up the benefits versus the risks and the risks are low, but it is still giving a drug for six months to children and most of them don’t need it. But the consequences of getting MDR-TB are so bad that we really want to prevent that,” he says.
There is also the question around what effect using a broad antibiotic as preventive treatment will have on the microbiome of children and how this might drive resistance to the fluoroquinolones. Seddon says stool samples were collected from the study participants to determine how the drug affected a child’s microbiome and the potential for driving resistance. These data will also be provided to the WHO.
“I think that the evidence base is now very strong on the basis of these two trials. I think you can really say the issue of whether levofloxacin prevents MDR-TB, we’ve put that to bed,” he says. “Are there going to be other studies? Yes. I think that this is not over, levofloxacin is not the perfect drug for preventive therapy.”
Marks adds to this saying: “An important next step for TPT in DR-TB contacts will be studies that evaluate regimens that are shorter than six months – a long time to take medication every day, which can often be challenging. Effective and safe shorter regimens are now being used for child contacts of drug-susceptible TB and we hope the same progress can be made for contacts of DR-TB.”
As Marks has already stated, currently there are no strong recommendations for MDR-TB prevention by the WHO. In the 2020 TB prevention guidelines, it recommends that the preventative treatment for MDR-TB should be either a fluroquinolone or other second-line agent. It does however caution that these recommendations are based on low-quality evidence. Because of this, it recommends that the preventative treatment for MDR-TB should be individualised, and it be based on the drug resistance profile of the presumed contact. The drugs levofloxacin and moxifloxacin- both fluoroquinolones – may be used unless resistance is suspected. For levofloxacin a dosing schedule for both adults and children are proposed in the document.
Subrayen says that in South Africa the 2019 guidelines for the management of Rifampicin Resistant-TB (RR-TB) does indicate the use of levofloxacin as prevention treatment. The guidelines state that for prevention treatment a fluoroquinolone-based, multidrug regimen is preferred (either levofloxacin and high-dose isoniazid or levofloxacin, high-dose isoniazid and ethambutol). And if exposed to fluoroquinolone-resistant RR-TB, then high-dose isoniazid could be given. Delamanid could be considered as a potential option in very select cases. A training manual published this year by the Department of Health suggests that levofloxacin can be given on its own – but also stresses that the evidence base is weak, something that TB-CHAMP has presumably now changed.
Future of TPT
Seddon says that in a perfect world the ideal TB preventive regimen would be a so-called Pan regimen that could be given for a short period of time, to someone who has been exposed to TB and it works regardless of whether they had been exposed to drug-susceptible or drug-resistant TB.
“There are studies planned to use other drugs for prevention. There’s a study planned to use bedaquiline for a month or two and potentially using injectables that you just have to give once every couple of weeks. So, I think although this [levofloxacin] is a good option now, and it’s probably the best option we have now, this is not perfect,” Seddon says.
The study Seddon is referring to is the BREACH-TB study, a phase three trial that will look at whether a one-month treatment regimen of oral bedaquiline could prevent all forms of tuberculosis. It would be given to people exposed to both drug-resistant and drug-susceptible TB, and in people with HIV infection, including pregnant women and children.
Responding to questions from Spotlight earlier this year when this study was announced in the press, Sonya Krishnan, Assistant Professor of Medicine at Johns Hopkins University and Eric Nuermberger, Professor of Medicine at Johns Hopkins University, said that they anticipate recruiting between 1600 and 2 00 people to take part in the study – they expect around 400 to 500 of these will be people living with HIV. They also said that the control arm will receive the current standard of care in the country rather than placebo.
When asked whether any South African study sites will be included in the clinical trial, they said, “We very much plan to partner with study sites in South Africa. South Africa has a long-standing history of research excellence in TB.”
“A shorter regimen that fights both drug-resistant and drug-susceptible TB would be a game-changer for those living with TB and get us closer to our shared goal of ending the epidemic by 2030,” said Dr. Atul Gawande, USAID assistant administrator for Global Health, in a statement on the study. “This clinical trial will lay the foundation for a remarkable innovation in our fight against TB: a single-dose, long-acting injectable medicine.”
Indeed, if the science and development pans out as Gawande suggests it might, the future of TB preventive therapy might well be an entire course of therapy delivered through a single injection rather than a month or more of pills. As indicated in an article in the journal Clinical Infectious Diseases, work is already underway on the development of bedaquline, isoniazid, and rifapentine long-acting injections – though the research is for now still only in mice.
‘Communities need to be involved’
Hesseling raises the point that when treating or preventing TB, more than just the latest research advancement is needed to improve TB outcomes.
“For me treatment follows diagnosis, actually strengthening healthcare services, making communities more aware and creating demand for kids accessing diagnosis, preventive treatment and appropriate treatment, is actually where it starts,” she says. “So tools are amazing, but we actually need to have strong, effective healthcare services and knowledgeable, empowered communities.”
Seddon adds to this saying that results like those from TB-CHAMP are “a bit irrelevant if it is all kind of top down, paternalistic coming from the researchers, coming from the health system”.
“We really need to generate a community demand for this, where individuals living in communities where this is a problem are calling for this and getting angry about this and demanding it in a way that I think we’ve achieved very well with the HIV community,” he says. “It’s all well and good doing the science and then even better to get it [levofloxacin] into a guideline, but until there’s real demand for from the end user, I think it’s only going to have a certain amount of reach.”
Note: The terms DR-TB and MDR-TB are used somewhat interchangeably – Spotlight uses DR-TB to refer to drug-resistant forms of TB in general and MDR-TB to refer specifically to TB that is resistant to isoniazid and rifampicin.
Republished from Spotlight under a Creative Commons Licence.
Source: Spotlight