Craters on Surface of Melanoma Cells Serve as Sites for Tumour Killing
Studying these craters could better assess immunotherapy’s success in treating solid tumours
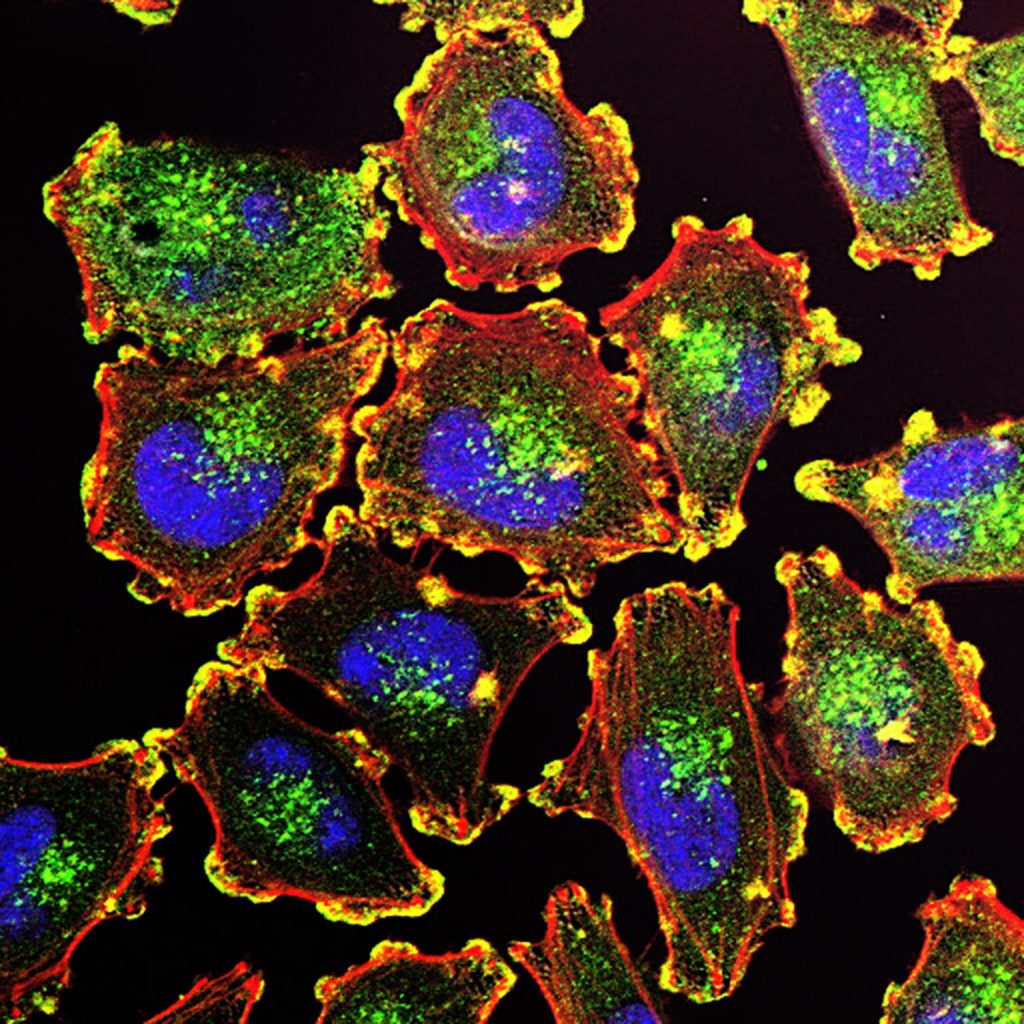
Like the surface of the moon, new research published today in Cell finds the existence of craters on the surface of melanoma cells that serve as immune hubs, becoming major sites for tumour killing. These craters could serve as good markers for immunotherapy success.
This research provides insight into a key function of immune check-point blockade (ICB) cancer therapy that was previously unknown. ICB works by re-activating CD8+ T cells against tumours which shrinks and eventually kills the cancer cells. However, what facilitates local tumour killing by the infiltrating CD8+ T cells has remained a mystery.
Using a zebrafish model, researchers were able to monitor the infiltrating CD8+ T cells for up to 24 hours as they moved through the 3D architecture of endogenous melanoma tumours. Zebrafish provide the only tumour model where continuous live imaging over a 24 hour time period is feasible.
“We found that rather than patrolling the entire tumour surface, CD8+ T cells aggregated in pockets on the melanoma border, forming prolonged interactions with melanoma cells,” says Leonard Zon, MD, Director of the Stem Cell Program at Boston Children’s Hospital and lead investigator of the study. “We termed these pockets Cancer Regions of Antigen presentation and T cell Engagement and Retention (CRATERs) and saw that, following immune stimulation, the CRATERs expanded and facilitated an effective immune response against the tumour.”
Zon, first author Aya Ludin, and the team also discovered CRATERs in human melanoma samples. Moreover, they saw similar structures in human lung cancer, indicating that CRATERs are likely not limited to melanoma and may form in other solid tumours.
To date, efficacy of therapeutic response to ICB therapy has been assessed mainly by estimating the degree of tumour necrosis and fibrosis. Indicators of CD8+ T cells infiltration has been associated with patient survival and treatment outcome, but direct evidence of effective immune cell-tumour cell interaction has been missing.
“Pending thorough clinical verification and taken together with other measurements, CRATERs may serve to more accurately assess the efficacy of an ongoing treatment and improve treatment outcomes,” said Zon.
The research team is now planning a prospective clinical trial to test if CRATERs are the best marker of ICB success.
Source: Boston Children’s Hospital via EurekAlert!