Aspergillus Flavus: From the ‘Curse of Tutankhamun’ to New Cancer Treatment

University of Pennsylvania-led researchers have turned a deadly fungus into a potent cancer-fighting compound. After isolating a new class of molecules from Aspergillus flavus, a toxic crop fungus linked to deaths in excavating ancient tombs, the researchers modified the chemicals and tested them against leukaemia cells. The result was a promising cancer-killing compound that rivals FDA-approved drugs and opens up new frontiers in the discovery of more fungal medicines.
“Fungi gave us penicillin,” says Sherry Gao, Presidential Penn Compact Associate Professor in Chemical and Biomolecular Engineering (CBE) and in Bioengineering (BE) and senior author of a new paper in Nature Chemical Biology on the findings. “These results show that many more medicines derived from natural products remain to be found.”
From Curse to Cure
A. flavus, named for its yellow spores, has long been a microbial villain. After archaeologists opened King Tutankhamun’s tomb in the 1920s, a series of untimely deaths among the excavation team fuelled rumours of a pharaoh’s curse. Decades later, doctors theorised that fungal spores, dormant for millennia, could have played a role.
In the 1970s, a dozen scientists entered the tomb of Casimir IV in Poland. Within weeks, 10 of them died. Later investigations revealed the tomb contained A. flavus, whose toxins can lead to lung infections, especially in people with compromised immune systems.
Now, that same fungus is the unlikely source of a promising new cancer therapy.
A Rare Fungal Find
The therapy in question is a class of ribosomally synthesised and post-translationally modified peptides, or RiPPs, pronounced like the “rip” in a piece of fabric. The name refers to how the compound is produced – by the ribosome, a tiny cellular structure that makes proteins – and the fact that it is modified later, in this case, to enhance its cancer-killing properties.
“Purifying these chemicals is difficult,” says Qiuyue Nie, a postdoctoral fellow in CBE and the paper’s first author. While thousands of RiPPs have been identified in bacteria, only a handful have been found in fungi. In part, this is because past researchers misidentified fungal RiPPs as non-ribosomal peptides and had little understanding of how fungi created the molecules. “The synthesis of these compounds is complicated,” adds Nie. “But that’s also what gives them this remarkable bioactivity.”
Hunting for Chemicals
To find more fungal RiPPs, the researchers first scanned a dozen strains of Aspergillus, which previous research suggested might contain more of the chemicals.
By comparing chemicals produced by these strains with known RiPP building blocks, the researchers identified A. flavus as a promising candidate for further study.
Genetic analysis pointed to a particular protein in A. flavus as a source of fungal RiPPs. When the researchers turned the genes that create that protein off, the chemical markers indicating the presence of RiPPs also disappeared.
This novel approach – combining metabolic and genetic information – not only pinpointed the source of fungal RiPPs in A. flavus, but could be used to find more fungal RiPPs in the future.
A Potent New Medicine
After purifying four different RiPPs, the researchers found the molecules shared a unique structure of interlocking rings. The researchers named these molecules, which have never been previously described, after the fungus in which they were found: asperigimycins.
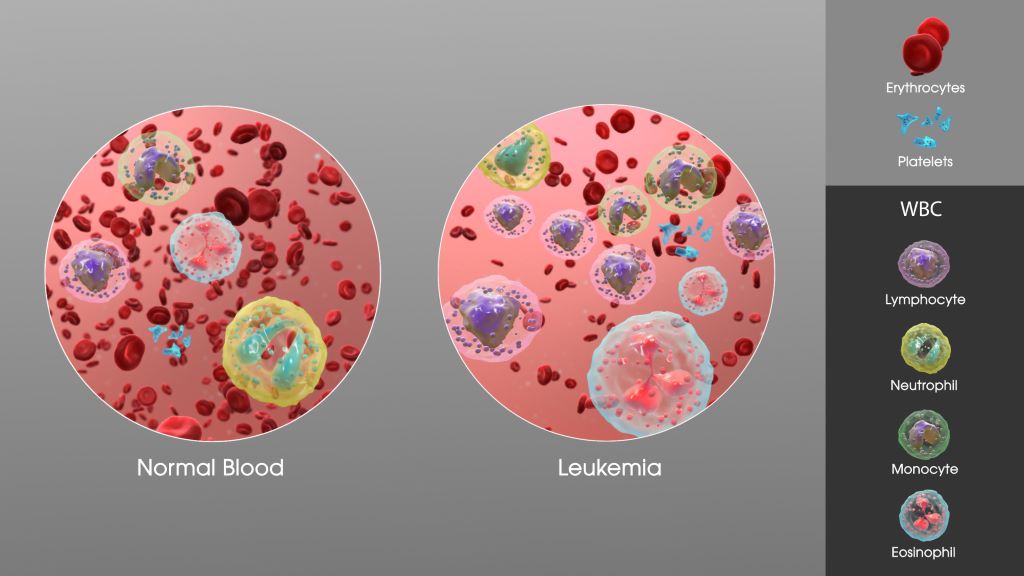
Even with no modification, when mixed with human cancer cells, asperigimycins demonstrated medical potential: two of the four variants had potent effects against leukaemia cells.
Another variant, to which the researchers added a lipid found in bees’ royal jelly, performed as well as cytarabine and daunorubicin, two FDA-approved drugs that have been used for decades to treat leukaemia.
Cracking the Code of Cell Entry
To understand why lipids enhanced asperigimycins’ potency, the researchers selectively turned genes on and off in the leukaemia cells. One gene, SLC46A3, proved critical in allowing asperigimycins to enter leukaemia cells in sufficient numbers.
That gene helps materials exit lysosomes, the tiny sacs that collect foreign materials entering human cells. “This gene acts like a gateway,” says Nie. “It doesn’t just help asperigimycins get into cells, it may also enable other ‘cyclic peptides’ to do the same.”
Like asperigimycins, those chemicals have medicinal properties – nearly two dozen cyclic peptides have received clinical approval since 2000 to treat diseases as varied as cancer and lupus – but many of them need modification to enter cells in sufficient quantities.
“Knowing that lipids can affect how this gene transports chemicals into cells gives us another tool for drug development,” says Nie.
Disrupting Cell Division
Through further experimentation, the researchers found that asperigimycins likely disrupt the process of cell division. “Cancer cells divide uncontrollably,” says Gao. “These compounds block the formation of microtubules, which are essential for cell division.”
Notably, the compounds had little to no effect on breast, liver or lung cancer cells – or a range of bacteria and fungi – suggesting that asperigimycins’ disruptive effects are specific to certain types of cells, a critical feature for any future medication.
Future Directions
In addition to demonstrating the medical potential of asperigimycins, the researchers identified similar clusters of genes in other fungi, suggesting that more fungal RiPPS remain to be discovered. “Even though only a few have been found, almost all of them have strong bioactivity,” says Nie. “This is an unexplored region with tremendous potential.”
The next step is to test asperigimycins in animal models, with the hope of one day moving to human clinical trials. “Nature has given us this incredible pharmacy,” says Gao. “It’s up to us to uncover its secrets. As engineers, we’re excited to keep exploring, learning from nature and using that knowledge to design better solutions.”
Source: University of Pennsylvania School of Engineering and Applied Science