Less Is More: Low-Dose Olanzapine Curbs Chemo-Induced Nausea Without the Sedation
A recent clinical trial demonstrates 5mg olanzapine’s safety and efficacy for chemotherapy-induced nausea and vomiting
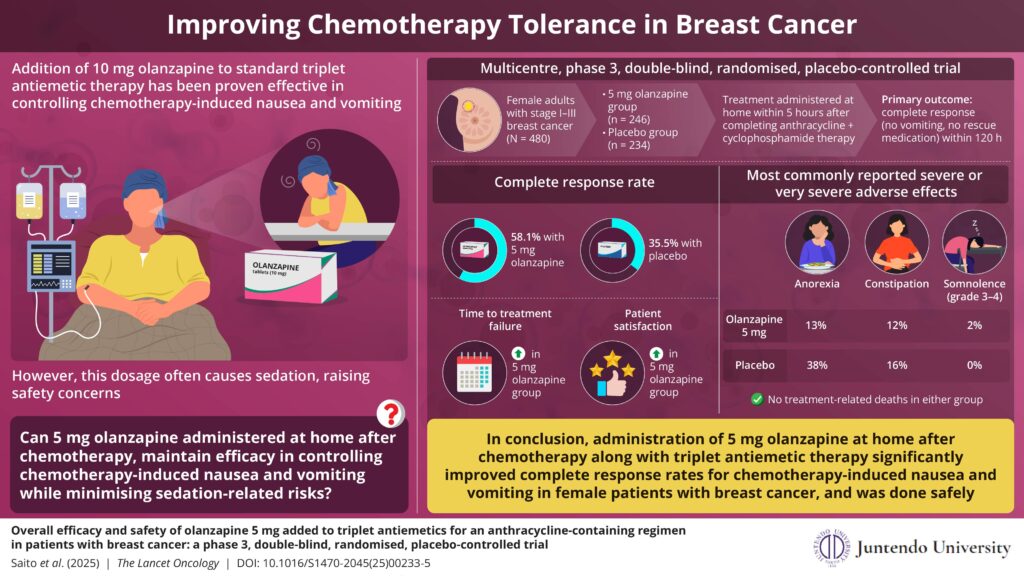
Chemotherapy-induced nausea and vomiting can severely impact patients’ quality of life and treatment adherence. In a major clinical trial, researchers from Japan tested whether a low, 5mg dose of olanzapine taken at home after chemotherapy could reduce these side effects without causing heavy sedation. The study found that this approach significantly improved outcomes compared to placebo, offering a safer, more affordable strategy that could reshape supportive cancer care, especially in outpatient and resource-limited settings.
Chemotherapy-induced nausea and vomiting are among the most distressing side effects of anti-cancer treatment, particularly for those receiving highly emetogenic regimens such as anthracycline plus cyclophosphamide combinations. This major side effect compromises a patient’s quality of life and willingness to continue therapy. Therefore, there is a crucial need to devise an effective antiemetic management approach for optimizing cancer care and patient well-being.
Against this backdrop, a new study, led by Professor Mitsue Saito and Dr. Hirotoshi Iihara from Japan, was made available online on June 17, 2025, and published in Volume 26, Issue 7 of the journal The Lancet Oncology on July 1, 2025, examined whether a 5mg dose of olanzapine taken at home after chemotherapy could reduce nausea and vomiting in patients with breast cancer while minimising the sedative effects associated with the standard 10mg dose.
“While multiple studies have examined 10mg of olanzapine and confirmed its effectiveness for nausea control, at this dose it often causes sedation, raising safety concerns,” explains lead author Prof Saito. “Beyond the commonly observed sedation, olanzapine at the 10 mg dose can cause serious adverse effects, including sedative effects such as daytime sleepiness and loss of consciousness.”
“The study was inspired in part by three patients with breast cancer who attended an antiemetic guideline meeting at MASCC 2015 in Copenhagen. They spoke about the burdensome sedative side effects of olanzapine, a concern that helped shape the trial’s patient-centred design,” says Prof Saito.
This phase 3, double-blind, placebo-controlled trial enrolled 500 female patients with breast cancer in Japan receiving outpatient anthracycline plus cyclophosphamide-based chemotherapy. Participants were randomly assigned to receive either olanzapine 5mg or placebo in combination with standard triplet antiemetic therapy (palonosetron, dexamethasone, and an NK-1 receptor antagonist). The olanzapine 5mg was taken at home after chemotherapy to help avoid sedation during hospital travel or treatment.
“This study uniquely investigates the timing of olanzapine 5mg administration, given within 5 hours post-chemotherapy administration and before the evening meal, to reduce sedation during hospital visits and transportation. This approach takes into account the onset of nausea and vomiting reported in previous studies. Among highly emetogenic chemotherapies, there is a significant difference between cisplatin, which usually requires hospitalisation for treatment, and other chemotherapies such as anthracycline-based regimens that are typically administered on an outpatient basis,” says Dr Iihara. The primary endpoint of the study was to investigate the proportion of patients achieving complete response, defined as no vomiting and no rescue medication use during the overall phase (0–120 hours post-anthracycline plus cyclophosphamide initiation).
The results demonstrated significant improvement, with 58.1% of patients in the olanzapine 5mg group achieving a complete response during the first 5 days after chemotherapy, compared to only 35.5% in the placebo group. Benefits also extended to delayed nausea and vomiting across a 7-day observation period.
While some patients reported drowsiness, the incidence of severe or very severe concentration impairment was low, occurring in 10% of patients in the olanzapine 5mg group vs 14% in the placebo group. Additionally, no major adverse events were observed in either group, indicating that there were no treatment-related deaths in either group.
The olanzapine 5mg dose offers an important financial and clinical advantage over the commonly used 10mg. By reducing side effects and cost, this strategy may make antiemetic treatment more accessible, particularly in lower-resource settings.
These new findings suggest that an olanzapine 5mg regimen, especially when administered after chemotherapy, can be just as effective, with fewer side effects. Although the study focused on Japanese women with breast cancer, the results are expected to influence international practices and future guideline updates.
In addressing both physical and financial toxicity and putting patients’ voices at the centre of the research, this trial represents more than a treatment tweak. It’s a step toward more humane, equitable cancer care.
Source: Juntendo University



