Synthetic Torpor has the Potential to Redefine Medicine
Nature is often the best model for science. For nearly a century, scientists have been trying to recreate the ability of some mammals and birds to survive extreme environmental conditions for brief or extended periods by going into torpor, when their body temperature and metabolic rate drop, allowing them to preserve energy and heat.
Taking inspiration from nature, Hong Chen, professor of biomedical engineering in the McKelvey School of Engineering and of neurosurgery at WashU Medicine, and an interdisciplinary team induced a reversible torpor-like state in mice by using focused ultrasound to stimulate the hypothalamus preoptic area in the brain, which helps to regulate body temperature and metabolism. In addition to the mouse, which naturally goes into torpor, Chen and her team induced torpor in a rat, which does not. Their findings, published in 2023 in Nature Metabolism, showed the first noninvasive and safe method to induce a torpor-like state by targeting the central nervous system.
Now, the team is in pursuit of translating induced, or synthetic, torpor into potential solutions for humans, such as when there is reduced blood flow to tissues or organs, to preserve organs for transplantation or to protect from radiation during space travel.
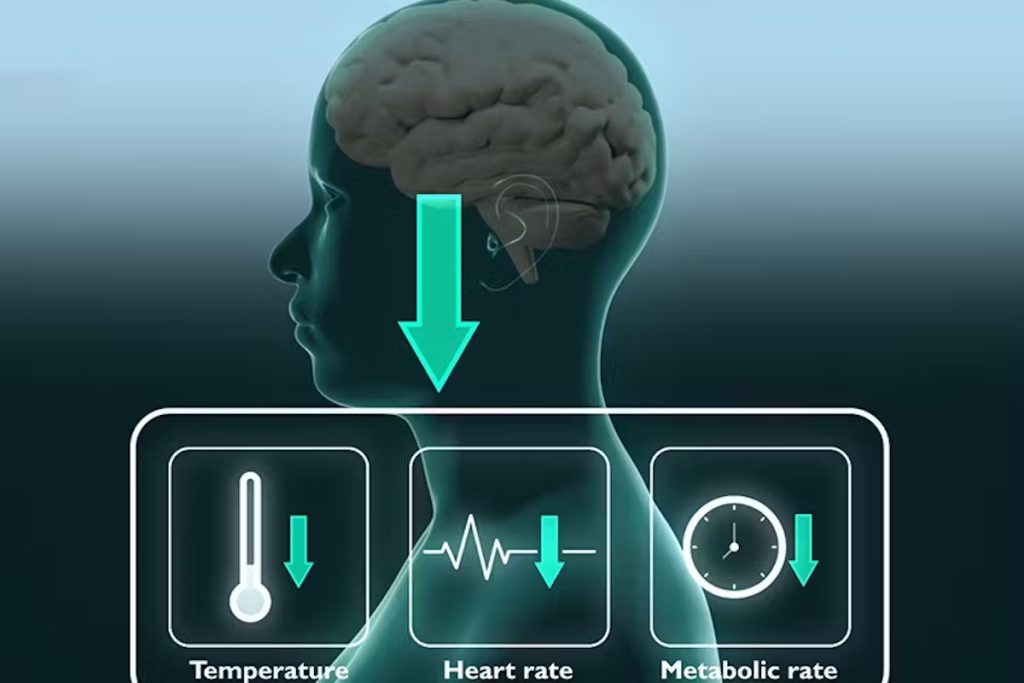
Conventional medical interventions focus on increasing energy supply, such as restoring blood flow to the brain after a stroke. Synthetic torpor seeks to do the opposite by reducing energy demand.
“The capability of synthetic torpor to regulate whole-body metabolism promises to transform medicine by offering novel strategies for medical interventions,” said Chen in a Perspectives paper published in Nature Metabolism July 31, 2025.
Synthetic torpor has been used successfully in preclinical models with medications and specialised targeting of the neural circuit, but there are challenges to adapting these methods for humans. Previous human trials with hydrogen sulfide were terminated early due to safety concerns.
“Our challenges include overcoming metabolic differences among animals and humans, choosing the correct dose of medication and creating ways to allow a reversible torpor-like state,” said Wenbo Wu, a biomedical engineering doctoral student in Chen’s lab and first author of the Perspectives paper, a collaboration between Chen’s team and Genshiro Sunagawa from the RIKEN Center for Biosystems Dynamics Research in Japan. “Collaboration among scientists, clinicians and ethicists will be critical to develop safe, effective and scalable solutions for synthetic torpor to become a practical solution in medicine.”
Chen’s team, including Yaoheng (Mack) Yang, who was a postdoctoral research associate in her lab and is now assistant professor of biomedical engineering at the University of Southern California, targeted the neural circuit with their induced torpor solution in mice. They created a wearable ultrasound transducer to stimulate the neurons in the hypothalamus preoptic area. When stimulated, the mice showed a drop in body temperature of about 3 degrees C for about one hour. In addition, the mice’s metabolism showed a change from using both carbohydrates and fat for energy to only fat, a key feature of torpor, and their heart rates fell by about 47%, all while at room temperature.
“Ultrasound is the only noninvasive energy modality capable of safely penetrating the skull and precisely targeting deep brain structures,” Chen said. “While ultrasound neuromodulation lacks cell-type specificity compared with genetic-based neuromodulation, it provides a noninvasive alternative for inducing synthetic torpor without the need for genetic modifications.”
Chen and her team indicate that synthetic torpor offers a promising therapeutic strategy with additional applications, including inhibiting tumour growth and potential development of new therapies for tau protein related diseases, such as Alzheimer’s disease. However, much remains unknown about how brain regions, peripheral organs and cellular pathways coordinate metabolic suppression and arousal. Researchers also need to study the long-term risks and potential side effects and call for more preclinical studies and technological innovations that will facilitate a dual approach, which would include modulating neural circuits associated with hypometabolism and influencing peripheral metabolic pathways through systemic interventions, such as with drugs or peripheral neuromodulation.
“Synthetic torpor is no longer just a theoretical concept – it is an emerging field with the potential to redefine medicine,” Chen said. “Bridging fundamental neuroscience, bioengineering and translational medicine will be key to overcoming current challenges and advancing synthetic torpor toward real-world applications. Synthetic torpor could transition from a scientific curiosity to a human reality through interdisciplinary collaborations.”
Source: Washington University McKelvey School of Engineering