Brain Training Game Offers New Hope for Drug-free Pain Management

The first trial of an interactive game that trains people to alter their brain waves has shown promise as a treatment for nerve pain – offering hope for a new generation of drug-free treatments.
You’re staring at a jellyfish drifting through inky black water on a screen. As your mind calms, the water turns turquoise. Nothing else seems to change, but the headset you’re wearing has picked up a subtle shift in your brainwaves and the game responds by altering the imagery. Now, for the first time, you can see your brain activity change. And by seeing it, you can practice making it happen again.
The game is part of PainWaive, a drug-free treatment for nerve pain developed by UNSW. Combining a game-like app and a brain-monitoring headset, PainWaive teaches users how to regulate the abnormal brain activity linked to chronic nerve pain, offering a potential in-home, non-invasive alternative to opioids.
The first trial of the treatment, led by Professor Sylvia Gustin and Dr Negin Hesam-Shariati from UNSW’s NeuroRecovery Research Hub, has delivered promising results, published in the Journal of Pain.
The study compared hundreds of measures across participants’ pain and related issues like pain interference before, during and after four weeks of interactive game play. Their brain activity was tracked via EEG (electroencephalogram) headsets, with the app responding in real time to shifts in brainwave patterns.
Three out of the four participants showed significant reductions in pain, particularly nearing the end of the treatment. Overall, the pain relief achieved by the three was comparable to or greater than that offered by opioids.
“Restrictions in the study’s size, design and duration limit our ability to generalise the findings or rule out placebo effects,” Dr Hesam-Shariati says.
“But the results we’ve seen are exciting and give us confidence to move to the next stage and our larger trial.”
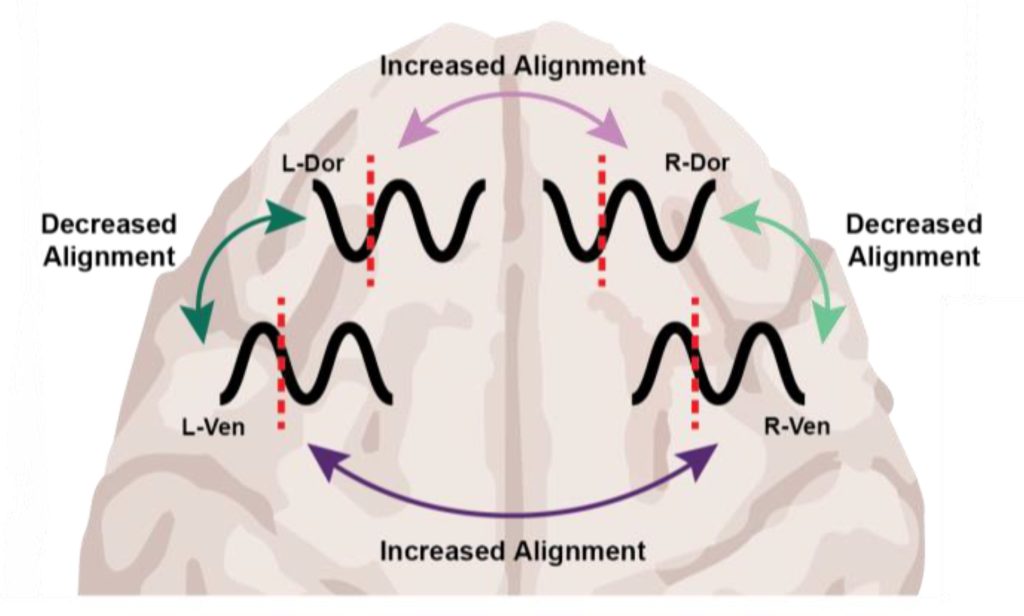
The PainWaive project builds on UNSW Professor Sylvia Gustin’s seminal research into changes in the brain’s thalamus – a central relay hub in the brain – associated with nerve (neuropathic) pain.
“The brainwaves of people with neuropathic pain show a distinct pattern: more slow theta waves, fewer alpha waves, and more fast, high beta waves,” Prof. Gustin says.
“We believe these changes interfere with how the thalamus talks to other parts of the brain, especially the sensory motor cortex, which registers pain.
“I wondered, can we develop a treatment that directly targets and normalises these abnormal waves?”
The challenge was taken up by an interdisciplinary team at UNSW Science and Neuroscience Research Australia (NeuRA), led by Prof. Gustin and Dr Hesam-Shariati, and resulted in PainWaive.
The four participants in its first clinical trial received a kit with a headset and a tablet preloaded with the game app, which includes directions for its use. They were also given tips for different mental strategies, like relaxing or focusing on happy memories, to help bring their brain activity into a more “normal” state.
The user data, meanwhile, was uploaded to the research team for remote monitoring.
“After just a couple of Zoom sessions, participants were able to run the treatment entirely on their own,” says Dr Hesam-Shariati.
“Participants felt empowered to manage their pain in their own environment. That’s a huge part of what makes this special.”
Initially, Dr Hesam-Shariati says, the team planned to use existing commercial EEG systems, but they were either too expensive or didn’t meet the quality needed to deliver the project. Instead, they developed their own.
“Everything except the open-source EEG board was built in-house,” says Dr Hesam-Shariati. “And soon, even that will be replaced by a custom-designed board.”
Thanks to 3D printing, Prof. Gustin says, the team has cut the cost of each headset to around $300 – a fraction of the $1000 to $20 000 price tags of existing systems.
The headset uses a saline-based wet electrode system to improve signal quality and targets the sensorimotor cortex.
“We’ve worked closely with patients to ensure the headset is lightweight, comfortable, and user-friendly,” says Prof. Gustin.
“Owning the technology offers us the potential to one day offer PainWaive as a truly affordable, accessible solution for at-home pain management, especially for those with limited access to traditional treatments.”
The team is currently focussed on a randomised controlled trial of the PainWaive technology, aiming to recruit 224 people with nerve pain following spinal cord injury.
It’s part of more than a dozen active collaborations between UNSW Science and the Centre for Pain IMPACT at NeuRA, all building on Prof. Gustin’s foundational research into the brain.
Among these is a clinical trial of an eHealth therapy, called Pain and Emotion Therapy, that was shown to reduce chronic pain by retraining the brain to process emotions more effectively.
Another major project, Project Avatar, is inspired by Prof Gustin’s discovery that half of people with complete spinal cord injuries still have touch signals reaching the brain – though the brain can’t identify them.
The trial uses immersive virtual reality and real-world touch stimulation to help the brain relearn how to feel.
“Many of our team are clinician-scientists, and we’re focused on developing practical treatments that can be integrated into the healthcare system,” says Prof Gustin.
“It’s incredibly inspiring to see results that help unlock the brain’s potential to heal itself and bring back hope to people living with pain.”
The researchers are now calling for participants to register their interest in two upcoming trials of the neuromodulation technology: The Spinal Pain Trial, investigating its potential to reduce chronic spinal pain, and the StoPain Trial, exploring its use in treating chronic neuropathic pain in people with a spinal cord injury.
Source: University of New South Wales