Astrocytes Identified as Hidden Culprit Behind PTSD
Excessive astrocytic GABA impairs fear extinction in PTSD, new drug target offers hope for treatment

Why do patients with post traumatic stress disorder (PTSD) often struggle to forget traumatic memories, even long after the danger has passed? This failure to extinguish fear memories has long puzzled scientists and posed a major hurdle for treatment, especially since current medications targeting serotonin receptors offer limited relief for only a subset of patients.
In a new discovery, scientists at the Institute for Basic Science (IBS) and Ewha Womans University have uncovered a new brain mechanism driving PTSD – and a promising drug that may counteract its effects. The research is reported in Signal Transduction and Target Therapy.
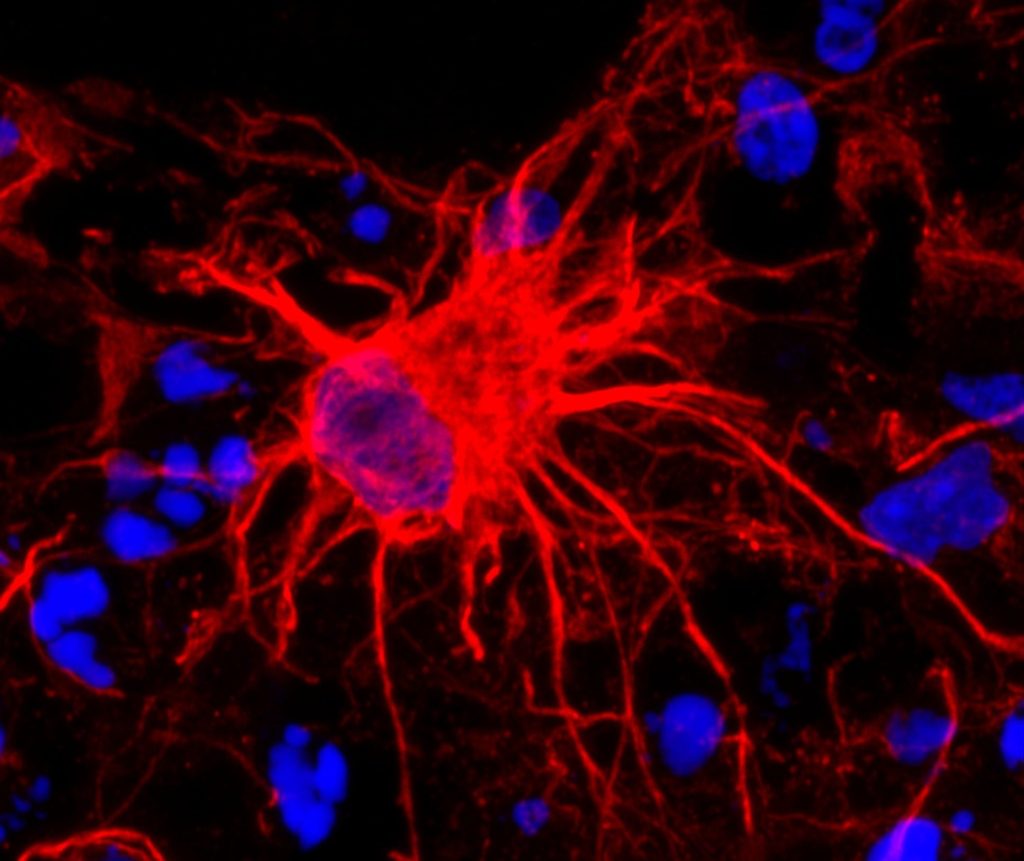
Led by Dr C. Justin Lee at the IBS Center for Cognition and Sociality and Professor Lyoo In Kyoon at Ewha Womans University, the team has shown that excessive GABA (gamma-aminobutyric acid) produced by astrocytes, which are star-shaped support cells in the brain, impairs the brain’s ability to extinguish fear memories. This deficit is a core feature of PTSD and helps explain why traumatic memories can persist long after the threat has passed.
Crucially, the researchers found that a brain-permeable drug called KDS2010, which selectively blocks the monoamine oxidase B enzyme responsible for this abnormal GABA production, can reverse PTSD-like symptoms in mice. The drug has already passed Phase 1 safety trials in humans, making it a strong candidate for future PTSD treatments.
PTSD remains difficult to treat, with current medications targeting serotonin pathways providing limited relief for many patients. The new study focused on the medial prefrontal cortex (mPFC), a region of the brain critical for regulating fear, and found that PTSD patients had unusually high levels of GABA and reduced cerebral blood flow in this area. These findings emerged from brain imaging studies of more than 380 participants. Importantly, GABA levels decreased in patients who showed clinical improvement, pointing to the chemical’s central role in recovery.
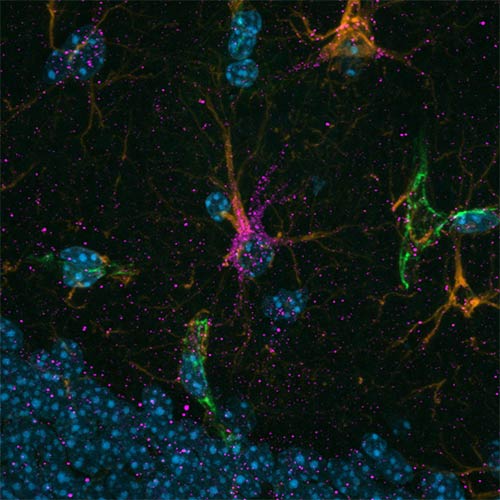
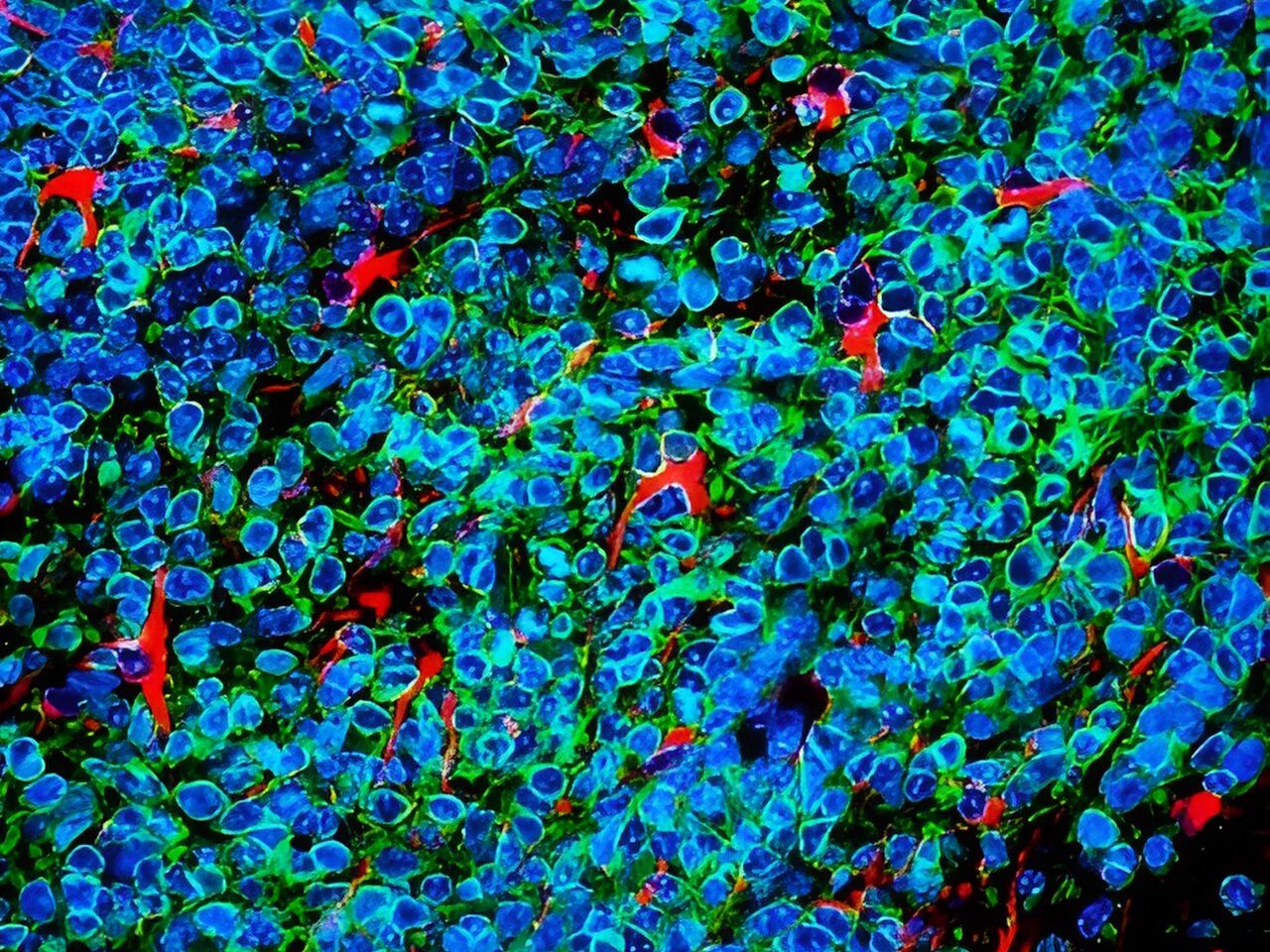
To uncover the origin of this excess GABA, the researchers examined postmortem human brain tissue and used PTSD-like mouse models. They discovered that astrocytes, not neurons, were producing abnormal amounts of GABA via the enzyme monoamine oxidase B (MAOB). This astrocyte-derived GABA impaired neural activity, blocking the brain’s ability to forget traumatic memories.
When the researchers administered KDS2010, a highly selective, reversible MAOB inhibitor developed at IBS, the mice showed normalized brain activity and were able to extinguish fear responses. The drug reduced GABA levels, restored blood flow in the mPFC, and re-enabled memory extinction mechanisms. The study thus confirms astrocytic MAOB as a central driver of PTSD symptoms, and MAOB inhibition as a viable therapeutic path.
A major challenge of the study was linking clinical findings in humans with cellular mechanisms in the lab. The researchers addressed this by applying a “reverse translational” strategy: they began with clinical brain scans and moved backward to identify the cellular source of dysfunction, then confirmed the mechanism and tested drug effects in animal models. This approach led to a new understanding of how glial cells – long thought to be passive – actively shape psychiatric symptoms.
“This study is the first to identify astrocyte-derived GABA as a key pathological driver of fear extinction deficit in PTSD,” said Dr Won Woojin, a postdoctoral researcher and co-first author of the study. “Our findings not only uncover a novel astrocyte-based mechanism underlying PTSD, but also provide preclinical evidence for a new therapeutic approach using an MAOB inhibitor.”
Director C. Justin LEE, who led the study, emphasized that “This work represents a successful example of reverse translational research, where clinical findings in human guided the discovery of underlying mechanisms in animal models. By identifying astrocytic GABA as a pathological driver in PTSD and targeting it via MAOB inhibition, the study opens a completely new therapeutic paradigm not only for PTSD but also for other neuropsychiatric disorders such as panic disorder, depression, and schizophrenia.”
The researchers plan to further investigate astrocyte-targeted therapies for various neuropsychiatric disorders. With KDS2010 currently undergoing Phase 2 clinical trials, this discovery may soon lead to new options for patients whose symptoms have not responded to conventional treatments.
Source: Institute for Basic Science