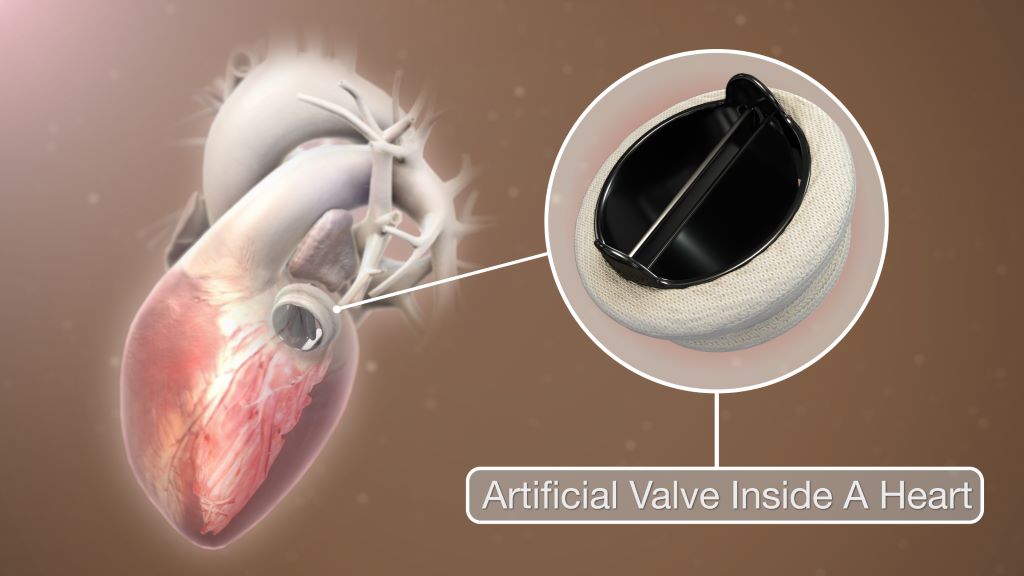
Heart Valve Which ‘Grows’ with Young Children Undergoing Preclinical Testing
Researchers at the University of California, Irvine have successfully performed preclinical laboratory testing of a replacement heart valve intended for toddlers and young children with congenital cardiac defects, a key step toward obtaining approval for human use. The results of their study were published recently in the Journal of the American Heart Association.
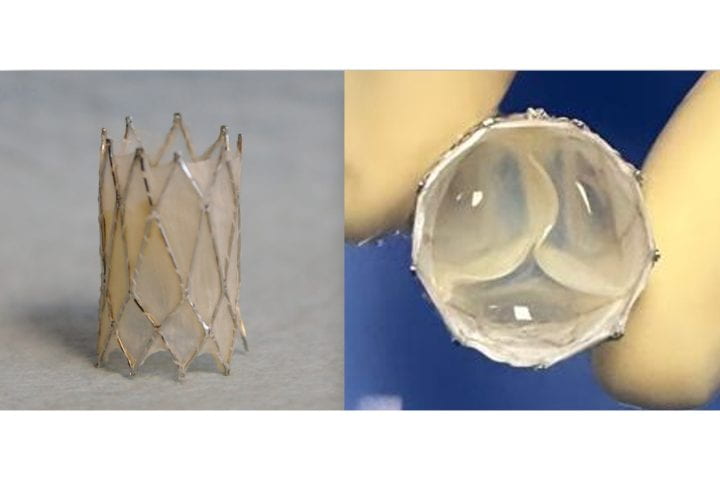
The management of patients with congenital heart disease who require surgical pulmonary valve replacement typically occurs between the ages of 2 and 10. To be eligible for a minimally invasive transcatheter pulmonary valve procedure, patients currently must weigh at least 20.4kg. For children to receive minimally invasive treatment, they must be large enough so that their veins can accommodate the size of a crimped replacement valve. The Iris Valve designed and developed by the UC Irvine team can be implanted in children weighing as little as 7.7 to 10kg and gradually expanded to an adult diameter as they grow.
Research and development of the Iris Valve has been supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute; and the National Science Foundation.
This funding has enabled benchtop fracture testing, which demonstrated the valve’s ability to be crimped down to a 3mm diameter for transcatheter delivery and subsequently enlarged to 20mm without damage, as well as six-month animal studies that confirmed successful device integration within the pulmonary valve annulus, showing valve integrity and a favourable tissue response.
“We are pleased to see the Iris Valve performing as we expected in laboratory bench tests and as implants in Yucatan mini pigs, a crucial measure of the device’s feasibility,” said lead author Arash Kheradvar, UC Irvine professor of biomedical engineering. “This work represents the result of longstanding collaboration between our team at UC Irvine and Dr Michael Recto at Children’s Hospital of Orange County built over several years of joint research and development.”
Congenital heart defects affect about 1% of children born in the United States and Europe, with over 1 million cases in the US alone. These conditions often necessitate surgical interventions early in life, with additional procedures required to address a leaky pulmonary valve and prevent right ventricular failure as children grow.
The Iris Valve can be implanted via a minimally invasive catheter through the patient’s femoral vein. The Kheradvar group employed origami folding techniques to compress the device into a 12-French transcatheter system, reducing its diameter to no more than 3mm. Over time, the valve can be balloon-expanded up to its full 20mm diameter.
This implantation method, along with the ability to begin treatment earlier in very young patients, helps mitigate the risk of complications from delayed care and reduces the need for multiple surgeries in this vulnerable population.
“Once the Iris Valve comes to fruition, it will save hundreds of children at least one operation – if not two – throughout the course of their lives,” said Recto, an interventional paediatric cardiologist at CHOC who’s also a clinical professor of paediatrics at UC Irvine. “It will save them from having to undergo surgical pulmonary valve placement, as the Iris Valve is delivered via a small catheter in the vein and can be serially dilated to an adult diameter and also facilitate the future placement of larger transcatheter pulmonary valves – with sizes greater than 20 millimetres, like the Melody, Harmony and Sapien devices – if needed.”
Source: University of California, Irvine