New Auditory Brainstem Implant Shows Early Promise
A new study co-led by Mass General Brigham researchers points to a promising new type of auditory brainstem implant (ABI) that could benefit people who are deaf due to Neurofibromatosis type 2 (NF2) and other severe inner ear abnormalities that prevent them from receiving cochlear implants. With further tests and trials, researchers hope it will provide a more effective treatment alternative than what is currently used.
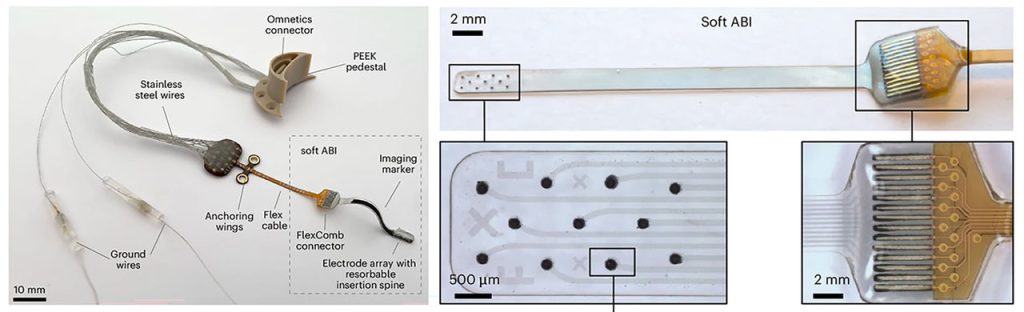
In the new research, published in Nature Biomedical Engineering, scientists at Mass Eye and Ear, a member of the Mass General Brigham healthcare system, collaborated with scientists at the École Polytechnique Fédérale de Lausanne (EPFL) in Geneva, Switzerland, to report on a new class of soft, flexible ABIs that were designed to address the limitations of those currently used. These implants bypass damaged auditory structures and directly stimulate the brainstem’s sound-processing region to restore auditory function.
The new ABI was borne out of a decade-long collaboration between Mass Eye and Ear and EPFL scientists. It features an elastic, multilayer construct that includes ultra-thin platinum electrodes and silicone, a novel design that allows it to conform closely to the brainstem’s curved surface.
Conventional ABIs that are sometimes used in patients with NF2 rely on stiff electrodes that struggle to conform to the curved surface of the cochlear nucleus in the brainstem. That limits their effectiveness to modest benefits, typically providing only basic sound awareness to aid lip reading. The design can also cause side effects like discomfort that discourages long-term use.
The novel, soft electrode design was developed using advanced thin-film processing techniques, allowing for closer contact and more precise stimulation. In preclinical tests conducted in Switzerland, two macaques received the implants and underwent several months of behavioural testing. Results showed the animals could consistently distinguish between different patterns of stimulation – which indicated high-resolution auditory perception, a promising sign for eventual human use.
“While cochlear implants are life-changing for many, there remains a group of patients for whom current technology falls short,” said study co-senior author Daniel J. Lee, MD, FACS, Ansin Foundation Chair in Otolaryngology at Mass Eye and Ear. “Our research lays the groundwork for a future auditory brainstem implant that could improve hearing outcomes and reduce side effects in patients who are deaf and do not benefit from the cochlear implant.”
Source: Mass Eye and Ear