Unusual Allies: Vagus Nerve Cells Emerge as Defenders Against Flu Damage
Study finds sensory cells that detect tissue damage, irritants also rein in harmful immune responses to protect the lungs
A group of nerve cells known for their role in detecting chemical irritation, tissue damage, heat, and pressure now emerge as critical defenders against the worst ravages of the flu caused by an overactive immune response, according to new research by scientists at Harvard Medical School and the Harvard T.H. Chan School of Public Health.
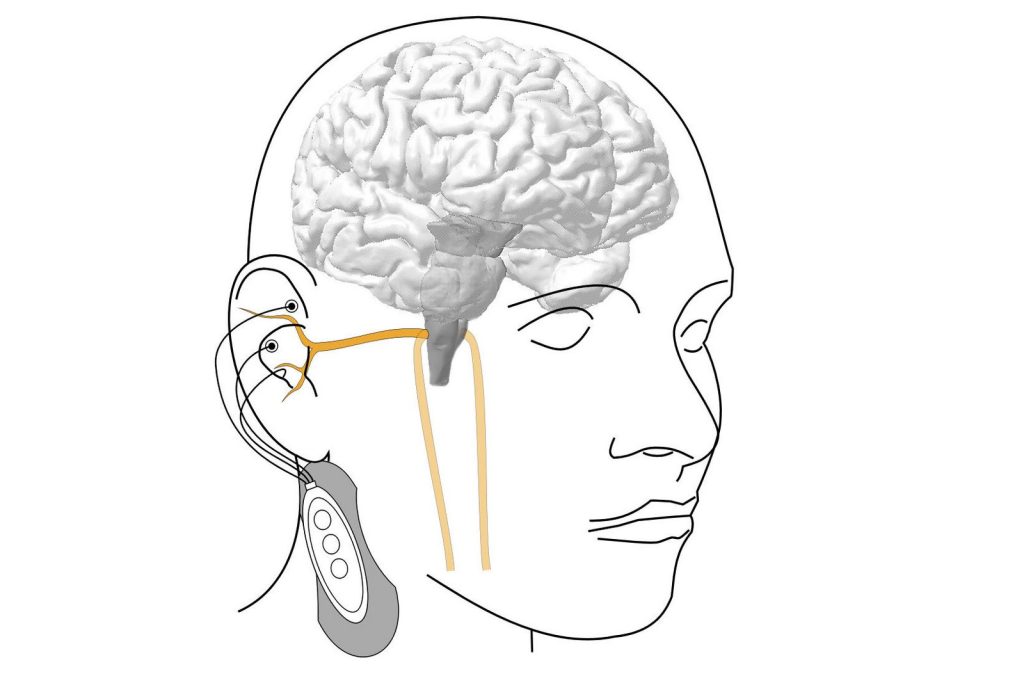
The cells, called TRPV1 vagal nociceptors, live in the vagus nerve, which sends signals from internal organs – including the heart, lungs, and gut – to the brain to help regulate heart rate, breathing, digestion, and other functions. In the lungs, these cells trigger the protective cough reflex that forces the airways to expel foreign particles, mucus, and other irritants.
But the new research, published in Science Immunology and conducted in mice, shows that in the setting of flu, these cells do much more – they rein in the immune system and avert the smoldering inflammation that often occurs in the aftermath of a viral infection and can injure healthy tissue.
Each year, the flu sickens millions and kills between 290 000 and 650 000 people worldwide, according to the World Health Organization. While the immune system helps fight off the virus, an excessive inflammatory response can inflict tissue damage and worsen illness. The findings are especially relevant in the wake of the COVID-19 pandemic, which revealed how an aberrant immune response following viral infection can sometimes lead to serious organ damage and even organ failure.
“Our research shows that the infected lung is a battleground where nerves and immune cells engage in a delicate dance to safeguard our health,” said co-senior study author Isaac Chiu, professor of immunology in the Blavatnik Institute at HMS. “Understanding this powerful neuro-immune signaling axis will be increasingly important as we design better ways to prevent and treat immune-mediated damage in viral infections, which can sometimes be worse than the direct damage caused by the virus itself.”
The findings, he added, raise the possibility that vagus nerve function may be one variable that explains why some people with the flu go on to develop long-lasting and devastating immune-driven damage in their lungs while others recover once the initial infection is resolved.
“Flu infections are highly variable in severity and there is a need to understand why certain groups of people, such as the elderly, experience worse disease,” said study first author Nicole Almanzar, a doctoral student in the Chiu Lab. “Our study demonstrates that the nervous system is actively involved in regulating the response of the lungs to infection, offering a new perspective on viral infections that could help explain why particular factors increase risk of severe infections.”
The new study illuminates the complex interaction between body and brain and adds to a growing body of research showing that the nervous and immune systems engage in highly orchestrated crosstalk during infection to modulate body defenses.
Previous research led by Chiu has revealed the intricate interplay between the two systems in bacterial pneumonia, flesh-eating disease, meningitis, pain, and itch.
One of Chiu’s earlier studies found that during bacterial infections of the lung, the same set of vagal nerve neurons suppressed the immune defences. In the new study, however, the immune-taming function of these cells worked to shield the lung from excessive damage during viral infection.
“Context clearly matters,” Chiu said.
Disabling the neurons worsened flu damage in the lungs
In a set of experiments, researchers exposed a group of mice with genetically disabled or chemically silenced TRPV1 neurons to influenza A virus. Mice without these nerve cells fared notably worse than mice with functioning TRPV1 cells. Even though the overall amount of virus in the lungs was the same in both groups, mice lacking TRPV1 neurons suffered more severe lung pathology, higher levels of harmful inflammation, and lower survival rates. Interestingly, Almanzar noted, even though the overall viral load remained the same, the spread of the virus within the lobes of the lungs was more pronounced in mice without these protective neurons.
The researchers also found that the absence of TRPV1 neurons altered the lung’s immune landscape. The lungs of mice lacking these neurons had an overabundance of neutrophils and macrophages – two types of immune cells that, in excess, can worsen tissue damage. At the same time, interferon signalling – one of the body’s most important viral-defence pathways – was seriously impaired in these immune cells.
In another experiment, the researchers used an antibody to deplete inflammation-driving cells in flu-infected mice lacking TRPV1 neurons. These animals had notably better survival than untreated mice lacking these protective neurons. The observation further underscores how nerve cells help prevent harmful immune reactions that can sometimes be more dangerous than the virus itself.
The researchers noted that they do not yet know precisely how TRPV1 neurons restrain the march of inflammatory cells at the molecular level – a question they plan to explore in subsequent work.
“The vagus nerve is powerfully controlling inflammation, but how it does so remains a mystery to be solved,” Chiu said. “But we’re excited that it plays such a strong role in viral infections.”
Harnessing the immune brake for therapy
Moving forward, this insight opens the door to exciting new avenues for therapy. Instead of only targeting the flu virus or dampening immune activity, the research team said, future treatments could mimic the function of nerve cells to ensure the delicate balance between protective and damaging immune responses is not thrown off.
The idea is not that far-fetched, the researchers said, noting that the FDA recently approved a therapy for rheumatoid arthritis by vagus nerve stimulation.
“Imagine if you could harness this brake to control inflammation in the lungs and beyond,” Chiu said. “By stimulating related circuits where the vagus nerve shuts down immune cells, one could envision treating immune-mediated dysfunction of many kinds, including that caused by viral infections.”
Source: Harvard Medical School