How the Brain Controls Its Blood Volume
Inhibitory neurons and astrocytes hold the key to sharper functional brain imaging –
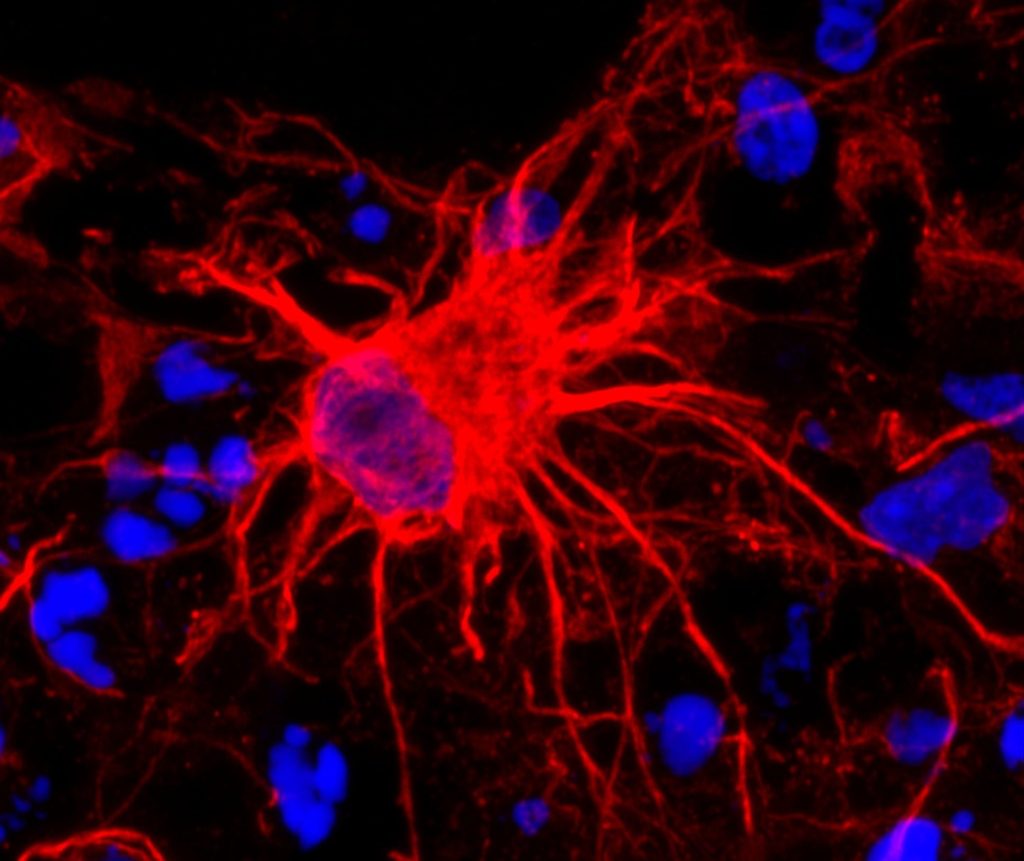
Researchers at the Center for Neuroscience Imaging Research within the Institute for Basic Science (IBS) have uncovered a two-step mechanism by which the brain regulates blood volume – a breakthrough with significant implications for how scientists understand and interpret functional magnetic resonance imaging (fMRI).
At the core of this study are somatostatin-expressing interneurons (SST neurons), a specialised type of inhibitory neuron. Among all neurons in the brain, approximately 15% are inhibitory neurons, but the role of these neurons in regulating cerebral blood volume has not been clearly elucidated in previous studies, with most of earlier research mainly focusing on the excitatory neurons. The new study by the IBS team sheds light on how inhibitory neurons interact with astrocytes, a type of support cell, to precisely control blood vessel dilation in the brain.
To unravel this complex process, the research team developed mouse models (e.g., SST-ChR2, SST-hM4Di) that allowed selective activation or inhibition of SST neurons. The researchers also used advanced neuroscience techniques in these mice. Specifically, they applied optogenetics and chemogenetics to precisely control the activity of these neurons. To observe the cellular and vascular responses, the team employed calcium imaging to visualise astrocyte activity, electrophysiology to measure neural signals, and intrinsic optical imaging (OIS) alongside ultra-high-field functional MRI (fMRI) to track blood volume dynamics with high spatial and temporal resolution.
Through these experiments, the team uncovered a two-step mechanism of vasodilation. In the early phase, SST neurons, when activated, released nitric oxide (NO) – a powerful vasodilator that triggered rapid and widespread expansion of nearby blood vessels. This was followed by a late phase, in which astrocytes became activated and induced slower, more localised vasodilation, especially during periods of prolonged sensory stimulation.

Notably, when SST neurons were silenced, the layer-specific precision of blood volume signals observed in fMRI was lost – a finding that links cellular activity directly to spatial specificity in imaging data.
“This two-step mechanism involving inhibitory neurons and astrocytes helps explain why high-resolution fMRI can distinguish activity between different layers of the brain cortex,” the researchers explained. “Our study fills a major gap in understanding how neural signals are translated into the blood volume changes we measure in brain imaging.”
The team also overcame several technical challenges – including distinguishing overlapping signals and timing differences – by integrating multiple imaging techniques and running extensive repeat experiments to validate the causal link between SST neuron activity and vascular responses.
Their findings not only advance basic knowledge of neurovascular coupling, but also open new avenues for investigating neurological and psychiatric conditions in which SST neuron function is impaired, such as Alzheimer’s disease, depression, and autism. The group plans to study disease-model mice to identify how disruptions in this neuron–astrocyte–vascular pathway affect blood volume, brain function, and fMRI signals.
Ultimately, this research lays the groundwork for improving the accuracy of brain imaging interpretation and may lead to better diagnostic and therapeutic strategies for brain disorders.
Source: Institute for Basic Science