Rare Congenital Paralysis Disease is Driven by Immune Cells
Study confirmed early-stage brain inflammation in mice

Patients with spastic paraplegia type 15 develop movement disorders during adolescence that may ultimately require the use of a wheelchair. In the early stages of this rare hereditary disease the brain appears to play a major role by over-activating the immune system, as shown by a recent study published in the Journal of Experimental Medicine. The study was led by researchers at the University of Bonn and the German Center for Neurodegenerative Diseases (DZNE). These findings could also be relevant for Alzheimer’s disease and other neurodegenerative conditions.
Spastic paraplegia type 15 is characterised by the progressive loss of neurons in the central nervous system that are responsible for controlling movement. Initial symptoms typically appear in late childhood, manifesting first in the legs in the form of uncontrollable twitching and paralysis. “What exactly causes these neurons to die is still not fully understood,” explains Professor Elvira Mass from the LIMES Institute at the University of Bonn. “In this study, we investigated the potential role of the immune system in this process.”
Professor Mass and Dr. Marc Beyer from the DZNE, together with Professor Ralf Stumm from University Hospital Jena, served as the study’s lead investigators, bringing together extensive experience to study this rare hereditary disease. The condition is triggered by a defect in the so-called SPG15 gene, which contains instructions for building a protein. But due to that defect, the protein cannot be produced.
Severe inflammation preceding the onset of cell damage
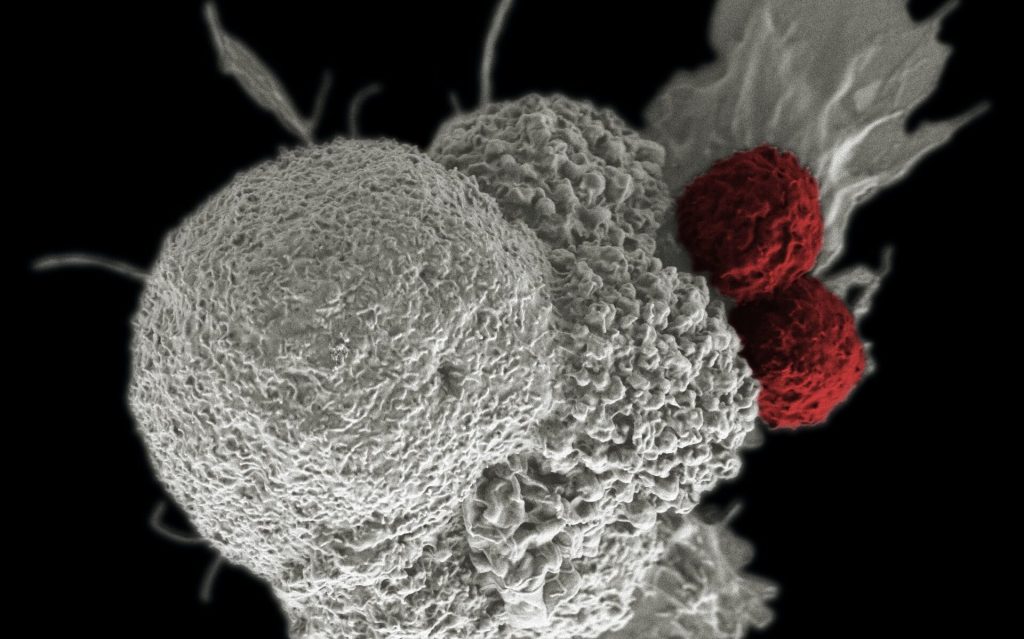
In their experiments the researchers used mice that shared the same genetic defect. “There was existing evidence that inflammatory processes in the brain play a role in development of the disease,” Dr Beyer explains, “So we studied microglia, which are the immune cells of the brain, and also whether immune cells in bone marrow are additionally involved in the inflammatory response.”
White blood cells form in bone marrow and can reach the brain via the bloodstream. Microglia, on the other hand, have already migrated to the brain during embryonic development. The researchers succeeded in specifically labelling the cells derived from bone marrow with a fluorescent dye. “This makes them distinguishable from microglia under a microscope,” Mass elaborates. “This allowed us to study the interaction between these two cell populations at the individual cell level.”
Analyses show that the microglia cells undergo dramatic changes in very early stages of the disease, long before any neuronal damage is identifiable. The cells are thereby altered into “disease-associated microglia.” These release messenger substances which, among other things, call for the help of cytotoxic “killer” T cells from the bone marrow that destroy other cells. The two cell types communicate with each other via signaling molecules, and their interplay drives the inflammatory process.
New therapeutic possibilities
“Our data suggest that the early stages of the disease are driven not by the loss of motor neurons but rather by the severe, early immune response,” Mass relates, “and that finding implies new therapeutic possibilities. Immune suppression drugs could potentially help slow progression of the disease.”
Inflammatory processes in the brain play an important role in Alzheimer’s and other neurodegenerative diseases. Spastic paraplegia is caused by entirely different conditions than dementia, but a very similar disruption of the immune system could be involved in dementia.
Source: University of Bonn